- Synthesis Spotlight
- Posts
- A Photocatalytic 1,2-Punch
A Photocatalytic 1,2-Punch
💡 From Weight Loss Hero to Alzheimer’s Zero

Monday 24th November – Sunday 30th November 2025 | Volume 2, Issue 45 |


Activation of Alcohols as Sulfonium Salts in the Photocatalytic Hetero-Difunctionalization of Alkenes
H. Zhao, D. Filippini, Y. Chen, A. Gallego-Gamo, L. S. Natrajan, L. R. E. Pantaine, C. Romano & D. J. Procter*
Nat. Chem. 2025 (DOI: 10.1038/s41557-025-02003-7) 🔓
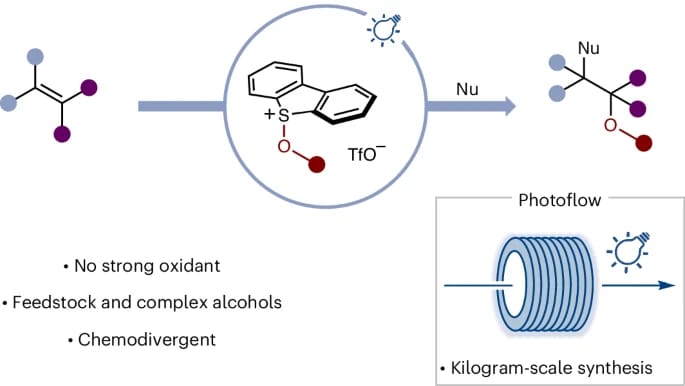
The authors describe a straightforward process in which both simple and complex alcohols can be converted under photocatalytic conditions to the corresponding alkoxy radicals—via the formation of alkoxy sulfonium salts—that react with alkenes en route to 1,2-diol and 1,2-amino-alcohol derivatives. The method can be easily adapted from laboratory to industrial, kilogram scale using a photoflow system.

A Unified Synthetic Approach to 2-Alkyl Azetidines, Oxetanes, Thietanes and Cyclobutanes from Unactivated Alkenes
L. Buck,† M. Pelosi,† S. Paul, E. Wheatley, A. Richardson, S. Ghosh, F. Sardelli & M. Silvi*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c11758) 🔓
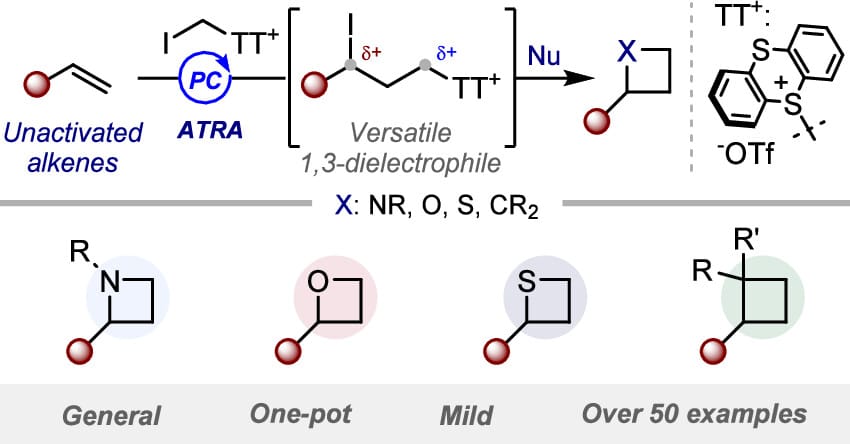
The authors introduce a general method to transform readily available unactivated alkenes into 2-alkyl four-membered rings. This strategy leverages a homologative alkene di-electrophilic activation, which first converts an alkene into a transient 1,3-iodo-sulfonium intermediate that can undergo sequential nucleophilic substitutions to promote the formation of azetidines, oxetanes, thietanes, and cyclobutanes.
Fluorocarbyne Insertion into Benzene Skeletons
C.-J. Wu,† M.-Y. Wang,† C. Wang,† J.-J. Zhi, L. Chen, Y.-G. Zou, H.-S. Wang, X. Hong* & W. Li*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c16875)
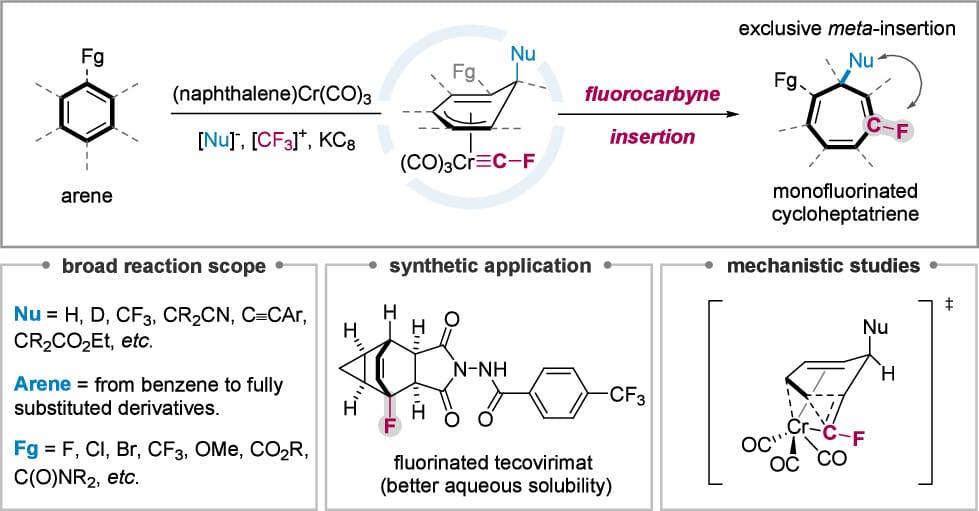
The authors report a fluorocarbyne-promoted skeletal editing that transforms simple benzenes into mono-fluorinated cycloheptatrienes. This fluorocarbyne insertion features broad functional-group tolerance, exclusive meta-insertion selectivity, and compatibility with a range of arene substrates. The method’s utility is evidenced by selective benzene activation within polyaromatics, effective late-stage modification of drug molecules, and its application to tecovirimat, affording a fluorinated analogue with an approximately three-fold enhancement in aqueous solubility.
Enantioselective Trifunctionalization of Terminal Alkynes
L. Yang, M. C. Detels & G. Lalic*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c15956)

The authors demonstrate palladium-catalyzed trifunctionalization of terminal alkynes using organoboranes and allylic carbonates as coupling partners. This transformation provides tetrasubstituted alkenes with excellent regioselectivity and diastereoselectivity. Moreover, regiodivergent, diastereo- and enantioselective incorporation of the allylic fragment provides access to a wide range of complex 1,4-diene products.
Redirecting Formate Delivery toward Alkenes: Markovnikov α-Carboxylation via Cobalt/Photoredox/Brønsted Acid Catalysis
S. Nandi,* S. Majumder, A. García-Eguizábal, I. Funes-Ardoiz* & D. Gryko*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c13504)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-nbxkt) 🔓
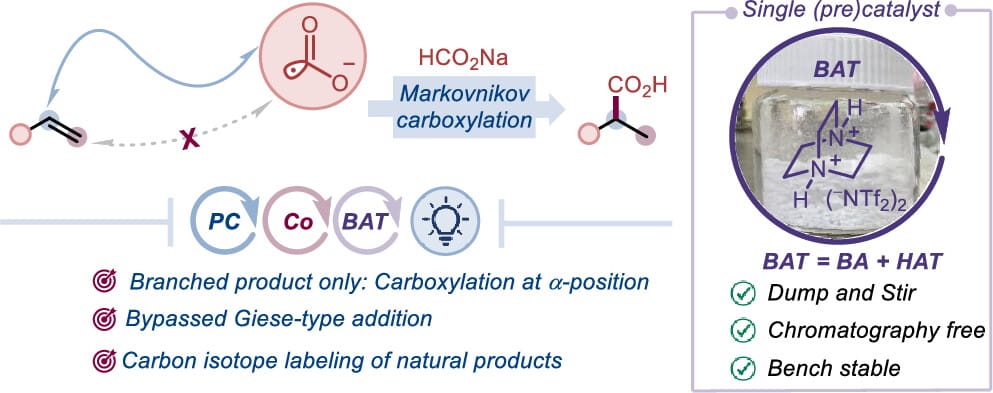
The authors report a photochemical approach for the selective synthesis of branched carboxylic acids using formate salts. This switch in regioselectivity from typical linear-selective carboxylation is achieved through the synergistic integration of an MHAT strategy with cobalt catalysis, a Brønsted acid, and photocatalysis. A bench- and air-stable N,N-diprotonated DABCO salt is introduced as a novel precatalyst that facilitates both catalytic cycles. The method accommodates a range of olefins, including bioactive compounds, and gives access to carboxylic acids bearing all-carbon quaternary centers.
Total Synthesis of Trigocherrins A and C
K. Takaoka, D. Matsubara, M. Matsumoto, M. Nagatomo, K. Hagiwara & M. Inoue*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c17272)
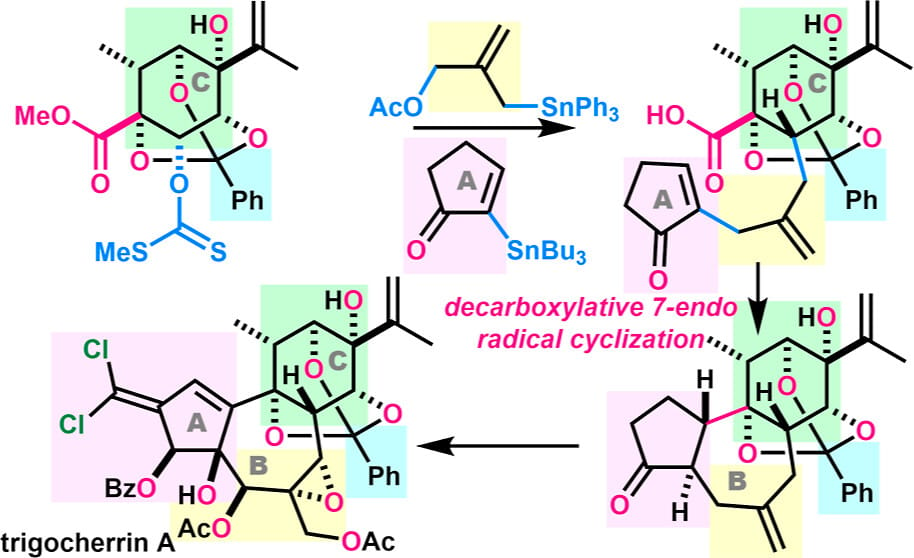
Trigocherrins A and C, isolated from an endangered tropical plant in New Caledonia, are rare daphnane diterpenoid orthoesters with a unique dichloroalkene moiety and 11 contiguous stereocenters. Here, the authors present the first total synthesis of trigocherrins A and C. The fully substituted C-ring was prepared from a ᴅ-ribose derivative, and sequentially coupled with a four-carbon unit and an A-ring by radical and Stille reactions, respectively. An Ir(III)-catalyzed photoinduced decarboxylative radical reaction stereospecifically cyclized the seven-membered B-ring. The AB-rings were adjusted by constructing the dichloroalkene and installing the multiple oxygen functional groups. Ultimately, the total syntheses were achieved in 37 total steps.
Convergent Total Synthesis of Papililone A via Pd-Catalyzed Alkenylation/Cyclization Cascade
X.-Q. Shan, X. Zhang, P.-F. Lian, B.-K. Guo, Y.-Q. Tu & S.-H. Hou*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c17278)
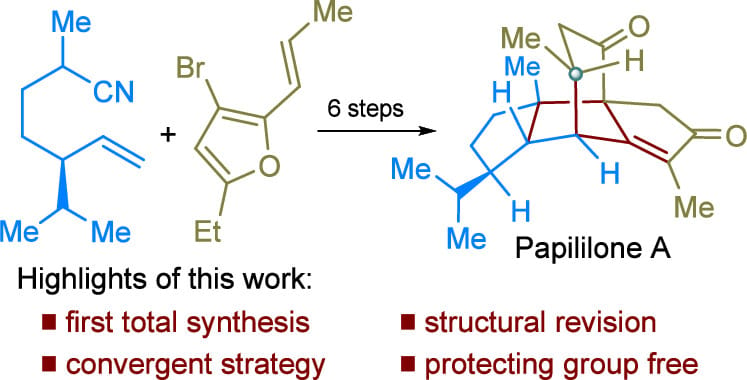
Papililone A is a polycyclic diterpenoid with anti-inflammatory activity, characterized by its intricate fused-bridged 5/5/5/6 tetracyclic skeleton and six contiguous stereocenters, including two adjacent bridgehead all-carbon quaternary centers. Here, the authors describe the first total synthesis of papililone A in a nine-step longest linear sequence (LLS) from commercial materials without the use of a protecting group, achieved through a cyclization cascade strategy.

Terpenoid Synthesis via Convergent Radical Annulation
A. L. Rerick, G. L. Barnes, F. Schneider, L. Oxenfart & P. S. Baran*
Angew. Chem. Int. Ed. 2025, Accepted (DOI: 10.1002/anie.202521852)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-cb46q) 🔓
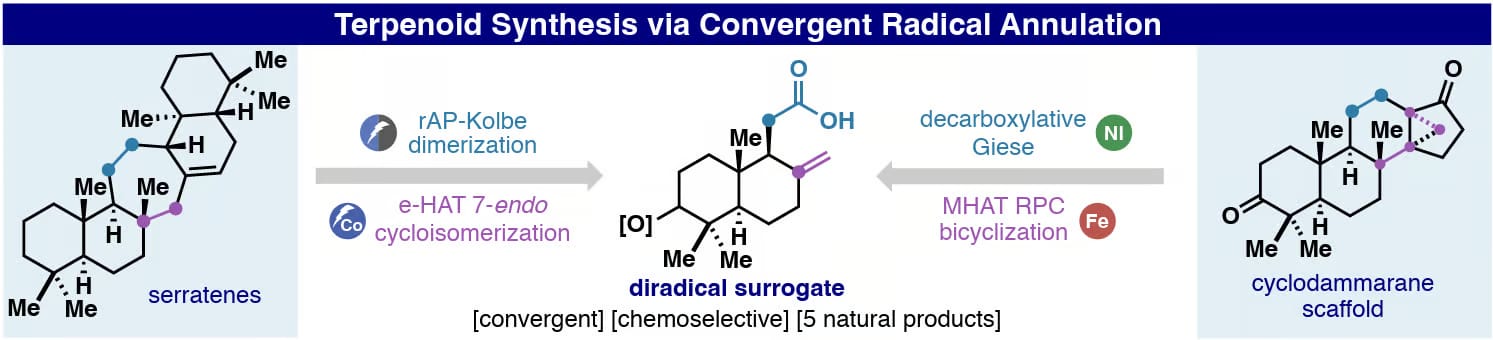
A convergent radical annulation strategy for the synthesis of complex terpenoids is disclosed. A 1,3-di-radical synthon enabled rapid C-ring annulations through sequential radical couplings to synthesize serratene and cyclodammarane scaffolds from sclareolide. These concise routes feature unique HAT radical cascades including a 7-endo-trig cycloisomerization and a radical/polar crossover bicyclization.

Metallaphotoredox Iron Catalysis Enables Direct Carbonyl-to-Carbene Conversion
P. S. Pedersen,† K. I. Burton,† S. H. M. Kaster,† A. L. Pace,† E. Lin, M. C. Bryan, T. M. Sodano, N. E. Intermaggio, C. B. Kelly & D. W. C. MacMillan*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-lsdnn) 🔓
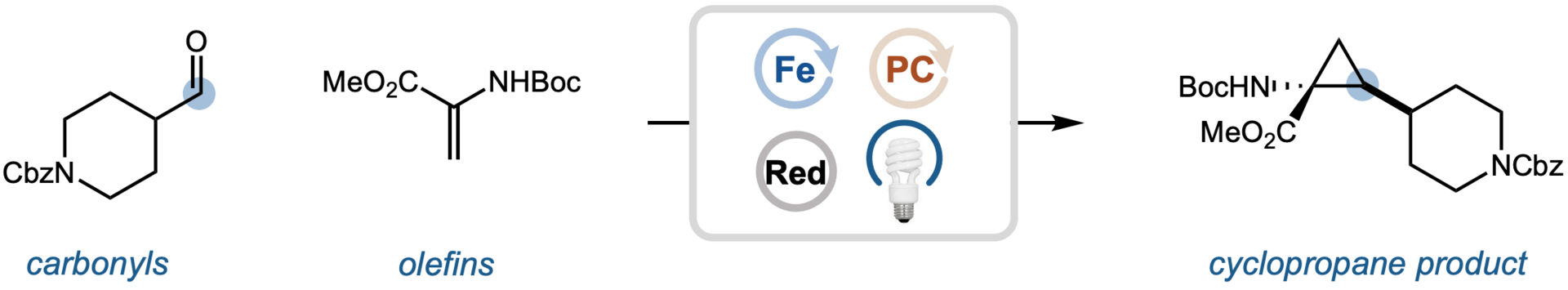
The authors report a photocatalytic strategy for direct carbene formation from unmodified carbonyl compounds using a low-valent iron system. The reaction proceeds through net oxidative addition of Fe(I) into the carbonyl bond, followed by α-protonation and α-elimination to generate a reactive Fe-carbene intermediate. This platform enables stereospecific cyclopropanation of diverse alkenes—including complex, drug-like, and peptide-derived substrates—under mild conditions, delivering highly functionalized, sp3-rich products.
ortho–meta and para–meta Isomerisation of Phenols
S. Malik, S. N. Ullal, J. D. Hart, M. Sodoor, P. A. Hume & P. S. Grant*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-f1853) 🔓

The authors present an approach to the para–meta and ortho–meta isomerisation of phenols, combining oxidative dearomatisation with photochemical rearrangement and reductive aromatisation. This method allows the transposition of neighbouring carbon atoms within the aromatic core, enabling access to challenging meta-functionalised phenols from readily available para- and ortho-isomers.
Scalable Photocatalytic Annulation of Primary Amines to δ-Lactams: Access to Privileged (Spiro)Piperidine Space
J. C. Turner-Dore, J. J. Bell-Tyrer, H. E. Askey, D. L. Richards, O. Polishchuk, O. P. Datsenko, J. A. Cadge, P. K. Mykhailiuk, M. Ciaccia & A. J. Cresswell*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-2cx97) 🔓
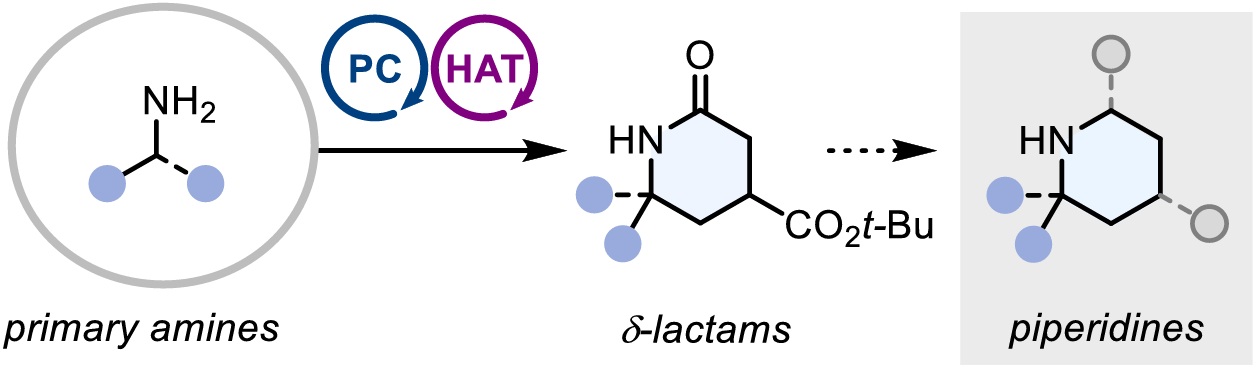
The authors disclose a general photoredox/hydrogen-atom transfer dual catalytic annulation that converts primary amines to α-mono- and α-disubstituted δ-lactams, versatile intermediates that provide streamlined access to diverse piperidines. The method proceeds under mild conditions, is scalable to 50 g in continuous flow, and affords products that undergo extensive downstream diversification.
Photoinduced Decarboxylative Dual Functional Group Transpositions
R. L. Pilkington & D. L. Priebbenow*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-grgz8) 🔓

The authors describe a thermodynamic approach to dual functional group transposition which relies on radical sorting across linear alkyl frameworks, promoted by visible light irradiation and decarboxylation. Suitably primed β-thioether or β-boryl carboxylic acids, first activated as oxime esters, then irradiated in the presence of an energy transfer photosensitiser, undergo radical S,N- or B,N-reconfiguration to generate to 1,2-iminosulfides or 1,2-iminoboronic ester products.
Molecular Reprogramming of Pyridines Unlocked by Photocatalysis
A. Cerveri,* M. Corrieri, C. Galbardi, M. Tegoni, L. Marchiò, M. Lanzi & G. Maestri*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-jkv8w) 🔓
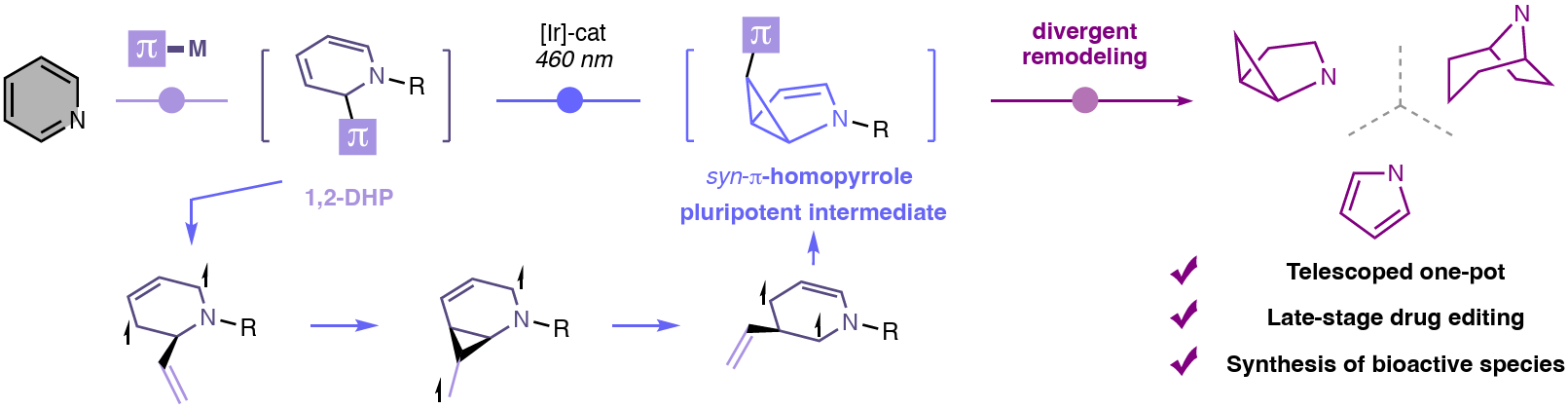
The authors introduce “molecular reprogramming” as a general strategy for the one-pot conversion of pyridines into diverse N-heterocycles, including homopyrroles, pyrroles, pyrrolidines, and tropanes. The key step of this sequence is a photocatalytic di-π-methane rearrangement, which furnishes syn-π-homopyrroles that readily undergo divergent modification. This approach enables the exploration of broad chemical-space, supports the late-stage derivatization of FDA-approved drugs, and provides access to privileged three-dimensional N-heterocycles, including bioactive compounds.
Isothioureas as Convenient Sulfur Donors in P–S Bond Formation
E. Courtney, G. L. Reber, N. Liasiuk & D. J. Jones*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-xdk7g-v2) 🔓

Isothioureas are demonstrated as practical sulfur donors for mild, one-pot P–S bond formation. Treatment with piperidine releases thiolate, which undergoes aerobic oxidation to the corresponding disulfide. Subsequent cross-dehydrogenative coupling with phosphine oxides affords phosphinothioates, phosphorothioates and phosphonothioates in high yields. The method proceeds under air, tolerates diverse organophosphorus functional groups, and avoids malodorous reagents or external oxidants, providing an efficient and operationally simple route to P–S-containing organophosphorus compounds.
👉️ Congratulations to David and his team! I worked with David during my post-doc, and he was an avid, early supporter of the newsletter. Funnily enough, this issue features publications from three PIs I’ve had the privilege of working alongside during my PhD and post-doc—congratulations to all.

Optimism, Meet Alzheimer’s Disease
💊 Optimism, Meet Alzheimer’s Disease. Just over a year ago, GLP-1 agonists such as semaglutide (Ozempic, Wegovy, Rybelsus) were riding a wave of optimism. These drugs weren’t just reshaping the treatment of obesity and diabetes, they were being tested for everything from kidney disease to alcoholism and even Alzheimer’s disease, prompting many to ask: “What can’t they do?”. At the same time, surging global demand for semaglutide turned Novo Nordisk into Denmark’s main economic engine. The pharmaceutical sector, with Novo at its centre, has driven more than half of the country’s recent GDP growth—a remarkable contrast to the UK, where the life sciences sector is “bleeding to death” (see our news story in Acids → Arynes).
This week, however, brought a reminder that even the most versatile drug classes have limits. Novo Nordisk reported that their oral semaglutide (Rybelsus) failed to slow disease progression versus a placebo in two large, two-year phase III trials involving roughly 3,800 patients with early-stage symptomatic Alzheimer’s disease, leading the company to cancel the planned extension phase. Although Novo noted improvements in certain Alzheimer’s-related biomarkers—and GLP-1 drugs have shown neuroprotective effects in animal studies as well as epidemiological evidence of reduced Parkinson’s and Alzheimer’s incidence in people with diabetes—these changes did not translate into meaningful clinical benefit.
Novo’s share price $NVO ( ▼ 0.51% ) dipped on the news, despite expectations for success being widely acknowledged as low.
“[W]e had a responsibility to explore semaglutide’s potential, despite a low likelihood of success”
Sadly, despite Denmark’s Novo Nordisk-related economic success, the company is cutting around 9,000 jobs globally (11% of its workforce). A stark contrast to 2023, when it accounted for roughly 20% of all new jobs created in the country.
💻️ Rising Stars in Medicinal Chemistry. Be sure to catch the German Chemical Society’s next Medicinal Chemistry webinar on Thursday 4th December, featuring Dr. Steinebach, Dr. Schroeder and Dr. Brewitz for a “Young Investigators Special with Round Table” with talks covering:
Leveraging induced-proximity modalities for drugging epigenetic proteins
Protein design as a new strategy in drug discovery
Broad-spectrum inhibitors of human 2-oxoglutarate-dependent dioxygenases as scaffolds for the rational development of selective inhibitors
That’s all for this issue! Have a great week and we’ll see you next Monday.

Reply