- Synthesis Spotlight
- Posts
- Acids → Arynes
Acids → Arynes
💡 Damning New Report Finds the UK’s Life Sciences Sector is "Bleeding to Death"

Note: Last week’s issue was slightly shorter than usual; however, this one’s a bit longer—there have been a number of excellent papers worth highlighting! Our apologies if a few great studies didn’t make it in this time; we’ve had to trim things to keep the email size within deliverability limits.
Monday 3rd November – Sunday 9th November 2025 | Volume 2, Issue 42 |


Myriad Aryne Derivatives from Carboxylic Acids
C. M. Seong,† S. S. Kargbo,† C.-L. Yu,† D. Gibney, J.-N. Boyn* & C. C. Roberts*
Nature 2025 (DOI: 10.1038/s41586-025-09830-1)

For making aromatic molecules, aryne intermediates have synthetic potential that rivals most functional groups. They readily react with nucleophiles, participate in pericyclic reactions, and activate inert σ-bonds. Despite their potential, arynes are currently used by a specialized community for mainly niche applications. The lack of widespread adoption of arynes is due to the undesirable means to generate them. Here, the authors report the design of an aryne precursor to overcome this prohibitive barrier. Readily available carboxylic acids are derivatized in a single step to a make a precursor which is then activated by blue light or by heat. Dozens of previously unknown aminated arynes, including pyridynes, are generated in this work.

Accelerating The Discovery of Multicatalytic Cooperativity
M. H. Sak, R. Y. Liu,* E. E. Kwan* & E. N. Jacobsen*
Nature 2025 (DOI: 10.1038/s41586-025-09813-2)
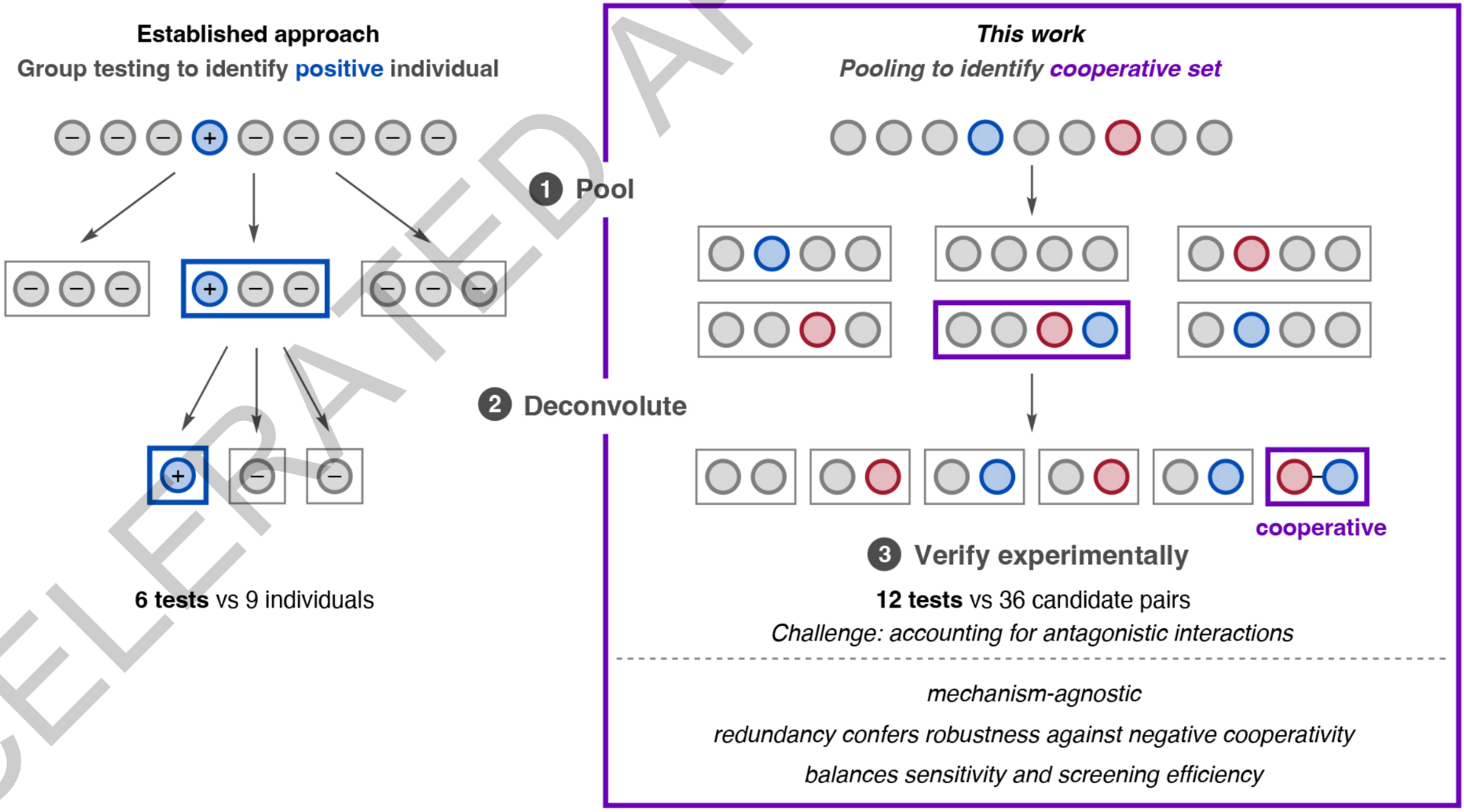
The authors describe a pooling–deconvolution algorithm, inspired by group testing, that identifies cooperative catalyst behaviors with low experimental cost while accommodating potential inhibitory effects between catalyst candidates. The workflow was validated first on simulated cooperativity data, and then by experimentally identifying previously documented cooperativity between organocatalysts in an enantioselective oxetane-opening reaction. The workflow was then applied in a discovery context to a Pd-catalyzed decarbonylative cross-coupling reaction, enabling the identification of several ligand pairs that promote the target transformation at substantially lower catalyst loading and temperature than previously reported with single ligand systems.

Direct Regioselective C-3 Halogenation of Pyridines
C. Li,† X. Li,† J. Li,† Z. Wang, D. Ouyang, N. Jiao* & S. Song*
Nat. Synth. 2025 (DOI: 10.1038/s44160-025-00915-3)
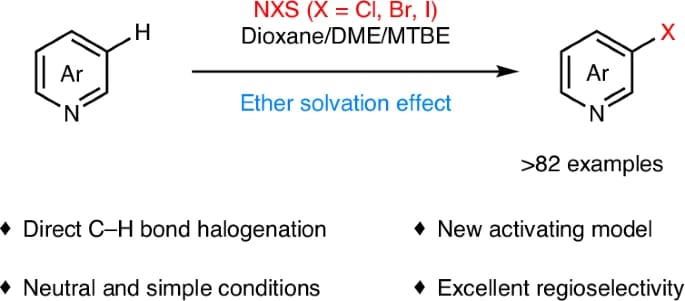
The authors describe a general direct regioselective C-3 halogenation of pyridines promoted by an ether solvation effect. This radical process enables the regioselective reaction to occur at the C-3 position of pyridines, rather than other aromatic C–H bonds, and can be applied to the late-stage halogenation of complex molecules.
Reductive Radical Chain Initiation Through the Thermal Generation of Carbon Dioxide Radical Anion
E. R. X. Lim, B. D. Cooper, M. Shanmugam, J. Da Luz, E. J. L. McInnes, C. Trujillo, J. J. Douglas & M. J. James*
Nat. Synth. 2025 (DOI: 10.1038/s44160-025-00919-z) 🔓

The authors report a general, thermally driven and scalable method for the reductive initiation of radical chains that involves reacting an inexpensive azo initiator with a formate salt to form a carbon dioxide radical anion. Substoichiometric quantities of this initiator system were used to form C(sp2 )–C(sp3 ), C(sp2 )–S, C(sp2 )–H, C(sp2 )–B and C(sp2 )–P bonds from complex (hetero)aryl halides, with high chemoselectivity and under transition-metal-free conditions.

Enantioselective Synthesis of (+)-Auriculatol A
J. K. Thompson, K. C. Youngblood, Y. H. S. Teh, C. M. Farley, Z. Zhang, S. C. Virgil & S. E. Reisman*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c17269) 🔓

A total synthesis of the grayanane diterpenoid (+)-auriculatol A is reported. The synthesis features a convergent coupling strategy that joins two fragments by a vinylogous Mukaiyama–aldol-type reaction and then constructs the 7-membered ring by a Ni-catalyzed enolate alkenylation. Key findings include the development of a chemoselective Ni-catalyzed intramolecular 1,2-addition to access the bicyclo[3.2.1]octane fragment and the use of electron-deficient olefin supporting ligands as uniquely effective for the Ni-catalyzed enolate alkenylation.
1,2-Oxygen Transposition on Arenes Enabled by Palladium/Norbornene Cooperative Catalysis
Q. Zhu, J. M. Taylor, X. Liu* & G. Dong*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c16468)
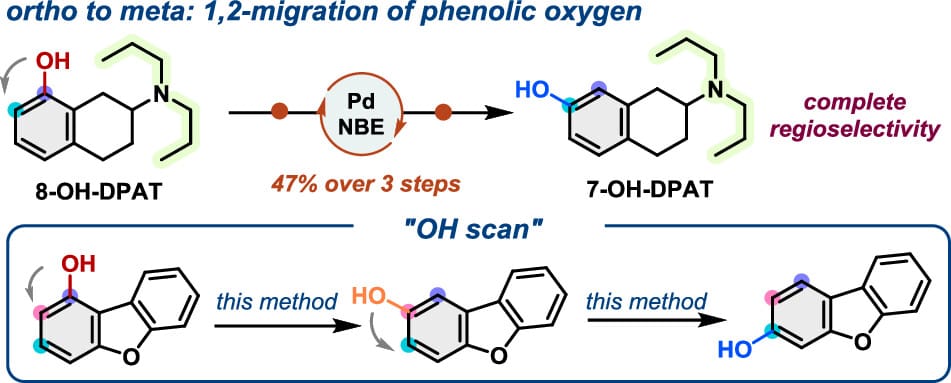
The authors report a palladium/norbornene cooperative catalysis-enabled 1,2-oxygen transposition on arenes. This method shows complete regioselectivity and tolerates substrates with various electronic properties and substitution patterns. While ortho-substituted substrates work best, preliminary success with ortho-unsubstituted phenols has been achieved, leading to an interesting “OH-scan” across three contiguous positions. Furthermore, late-stage 1,2-transposition of phenolic oxygen in three pharmaceutically relevant compounds was successfully demonstrated.
Enantioselective N-Heteroaryl C–H Functionalization for Piperidine-Azine Conjugation: Alkynes as Allylmetal Pronucleophiles in Dearomative Addition-Hydrogen Auto-Transfer
S. Lee, Y. S. Teoh,‡ P.-P. Xie,‡ E. M. McManus,‡ B. I. Gonzalez,‡ T. A. Claggett, P. Liu & M. J. Krische*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c15097)

The first enantioselective N-heteroaryl C–H functionalizations via dearomative addition-hydrogen auto-transfer are described. Using a ruthenium catalyst assembled from RuHCl(CO)(PPh3)3, (R)-Cy-BINAP and a piperidine- or azetidine-containing alkyne as an allylmetal pronucleophile, electron-deficient N-heteroaromatic compounds are converted to branched products of C–H allylation with uniformly high levels of enantioselectivity.
Copper-Catalyzed Enantioselective Synthesis of N-Heterocycle-Substituted Quaternary Carbon Stereogenic Centers
M. Seo & H. Kim*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c13377)

The authors report a copper-catalyzed enantioselective hydropyridylation of 1,1-disubstituted allenes with 2-halopyridines that proceeds via an SNAr pathway. This transformation enables the efficient synthesis of α-quaternary N-heterocyclic compounds with a broad substrate scope in high yields (up to 96%) with excellent enantioselectivities (up to 99% e.e.). The methodology is compatible with various functional groups and N-heterocycles and can be extended to copper-catalyzed boropyridylation, affording versatile alkenylboronates.
Development, Application, and Mechanistic Interrogation of a Dual Ni Catalysis Approach to Photoredox-Based C(sp3 )–C(sp3 ) Cross-Coupling
E. M. Bucci,† M. A. Perea,† R. F. Lalisse, P. Mukherjee, T. J. Raab, L. K. Valloli, D. S. Min, M. J. Bird,* O. Gutierrez* & A. G. Doyle*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c10906)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-xjl6p) 🔓

The authors report the development, application, and interrogation of a conceptually novel mechanistic framework for C(sp3 )–Me bond formation using two distinct Ni catalysts capable of cross-coupling sterically and electronically diverse alkyl halides (chlorides and bromides) with methyl radical generated photocatalytically from benzaldehyde dimethyl acetal. Furthermore, by modifying the alkyl substituents on the acetal coupling partner, cross-couplings beyond methylation were demonstrate to access an array of 1°–1° and 1°–2° alkyl–alkyl bonds.
Total Synthesis of (+)-Resiniferatoxin via a Pyridinium Salt Photorearrangement
H. Yu, L. Kong, J. Qin, Z. Wang & T. Luo*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c16410)

The authors report a total synthesis of (+)-resiniferatoxin (RTX) that leverages both the ground- and excited-state reactivity of pyridinium to assemble its highly oxidized 5/7/6-fused tricyclic core. A key strategic element is the development of a practical protocol for the in situ formation of pyridinium trifluoromethanesulfonate salts via mild methylation of densely substituted pyridines, followed by a ring-contracting photorearrangement. Elaboration of the resulting aziridine intermediate provides gram-scale access to advanced intermediates, which represents a modular route to highly functionalized five-membered carbocycles embedded in fused-ring scaffolds, thereby facilitating biological studies and analogue development of RTX.

C(sp2 )–C(sp3 ) Cross-Coupling Enabled by Alkyl Radical Capture at Isolable, Low-Spin (S = 1/2) Cobalt(II)–Monoaryl Catalysts
K. Choudhary, B. Paul & L. R. Mills*
ACS Catal. 2025, ASAP (DOI: 10.1021/acscatal.5c07125)

A cobalt(II) catalyst supported by the ligand 2-(diphenylphosphino)phenol (P,O) was developed for the C(sp2 )–C(sp3 ) Negishi arylation of alkyl(pyridyl)sulfones, which are bench-stable, non-organohalide C(sp3 )–S electrophiles. Employing the catalyst generated in situ from 5 mol % P,O ligand and 5 mol % cobalt(II) bromide, a variety of (hetero)aryl C(sp2 )–C(sp3 ) products were synthesized derived from primary and secondary alkyl sulfones, including difluoromethylation using 2-((difluoromethyl)sulfonyl)pyridine, and cross-coupling of sulfone derived from the thiol-containing ACE inhibitor captopril.

Direct Deoxyarylation of Non-Activated Alcohols
Q. Pan, R. Yu, Z. Cai & Y. Chen*
Angew. Chem. Int. Ed. 2025, Early View (DOI: 10.1002/anie.202520148)

A direct deoxygenative strategy for the cross-coupling of alkyl alcohols with various aryl electrophiles has been developed. The substrate alcohols require no pre-activation and the reaction occurs in a single step, representing the first direct deoxygenation arylation of non-activated alkyl alcohols.
Aminative Suzuki–Miyaura Coupling: Tackling Alkyl Nucleophiles and Addressing the Viability of the Electrophile-First Mechanism
S. Z. Li & R. Y. Liu*
Angew. Chem. Int. Ed. 2025, Early View (DOI: 10.1002/anie.202518260)

The Pd-catalyzed aminative coupling of primary and secondary alkyl boronic esters with aryl (pseudo)halides is reported. DFT calculations and mechanistic experiments show that the first C–N bond formation may take place through an unusual dyotropic rearrangement of a LPd(Ar)NHX complex (X = OPOPh2).

cis-Selective Direct Halocyclopropanation of Alkenes Mediated by Nucleophilic Cobalt Photocatalysis
J. H. G. Teye-Kau, M. Pauze & S. P. Pitre*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-lflm6) 🔓

The authors report a Vitamin B12-photocatalyzed approach for the direct synthesis of 1,2-disubstituted halocyclopropanes starting from alkenes and simple haloforms under visible-light irradiation. This method yields a diverse range of 1,2-disubstituted halocyclopropanes with high cis-selectivity, providing efficient access to these traditionally difficult to prepare stereoisomers.

Direct Access to Chiral Nitrogen-Rich (semi-)Saturated Heterocycles
M. Pierau,† M. J. Karrasch,† P. Hartmann, C. G. Daniliuc, A. Hamza* & F. Glorius*
Chem 2025, Online Now (DOI: 10.1016/j.chempr.2025.102817) 🔓
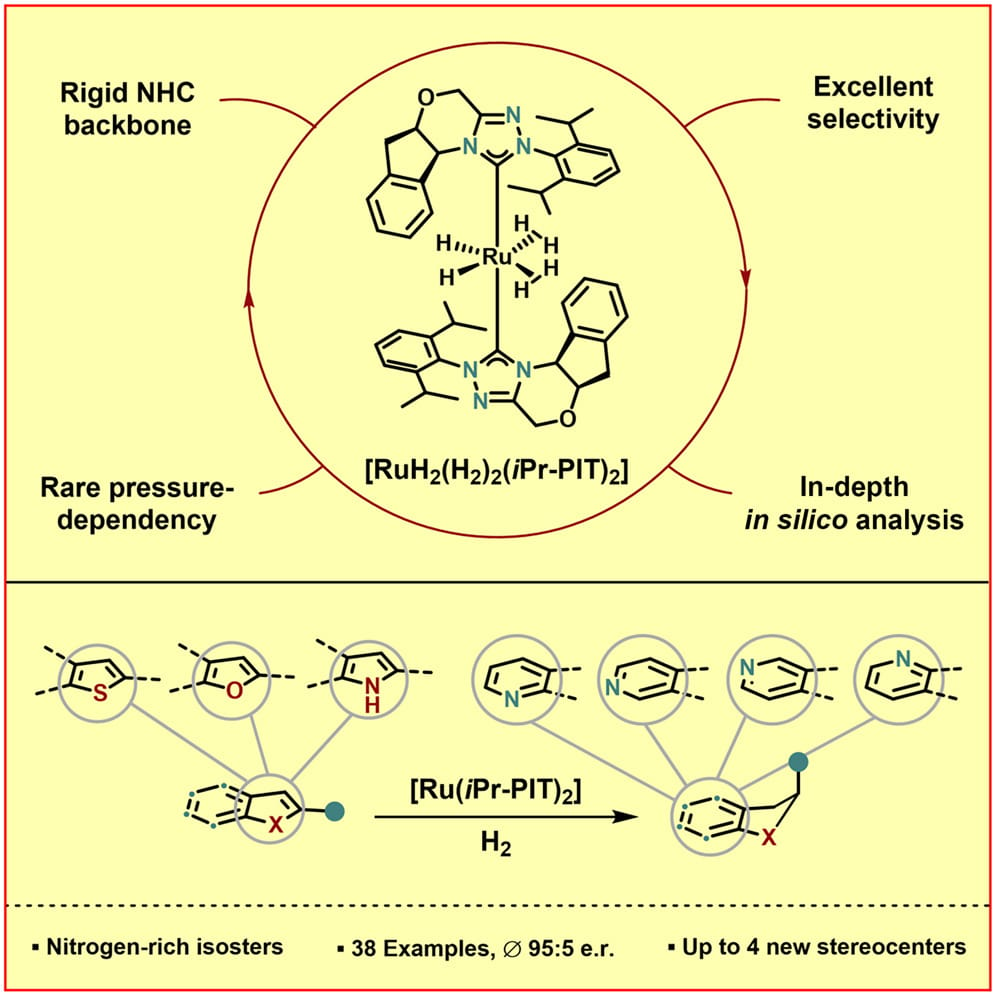
The authors report an efficient method for the synthesis of chiral (semi-)saturated pyridine-fused heterocycles and their respective N-permutations by enantioselective arene hydrogenation with a newly developed ruthenium catalyst. Versatile and highly valuable product motifs were obtained, including pyridine- and piperidine-fused scaffolds with up to four newly formed stereocenters, of which several have not been previously reported.

📰 Science 🤝 Newsletters. Some of you might remember when we highlighted Plenty of Room, a brilliant newsletter that delivers weekly insights into the frontiers of AI-driven protein design and DNA nanotechnology.
Well, science-focused newsletters are continuing to grow in popularity, helping researchers stay up to date with the latest developments in their fields. One excellent new addition I’m happy to highlight is Simply Drug Discovery, another Beehiiv-powered publication dedicated to providing clear, practical insights and resources in medicinal chemistry and drug discovery. Expect useful guides, mini-reviews of fundamental concepts, and explorations of emerging topics.

Bleeding To Death
🩸 Bleeding to death. The UK’s science and technology sector is “bleeding to death”, according to a damning new report from the House of Lords Science and Technology Committee, which warns that the nation’s research base and innovation ecosystem are at breaking point.
The report, titled “Bleeding to Death: The Science and Technology Growth Emergency”, paints a bleak picture: world-class universities and start-ups are being undermined by chronic underinvestment, punishing visa costs, and the flight of high-growth companies overseas. Without immediate and radical reform, the Lords warn, the UK risks becoming an “incubator economy”, nurturing early innovation only for it to mature (and profit) elsewhere.
The life sciences sector is amongst the hardest hit. Pharmaceutical giants including MSD, AstraZeneca, Eli Lilly, and Sanofi have recently scaled back or cancelled UK investments, citing the government’s lack of long-term commitment to R&D and ongoing disputes over drug pricing.
“The major pharmaceutical companies have said to me, in one form or another, that they are out and are not coming back”
The consequences are already visible. As we reported earlier this year, MSD’s decision to scrap its £1 billion Discovery Centre in London—along with laying off 125 highly skilled staff—marked a low point for UK life sciences. Similar retrenchments from AstraZeneca and others underscore a widening competitiveness gap with Europe and the US.
The report notes that while the UK hosts four of the world’s top ten universities, it has only three of the top 100 industrial R&D spenders—and none in the top ten. We’re training the next generation of innovators, entrepreneurs, and captains of industry, only to see them graduate into an economy that undervalues, underfunds, and undercuts their potential.
One problem is that UK pension funds, often fragmented and risk-averse, now allocate less than 5% of their assets to domestic equities, compared with 50% in the late 1990s, starving UK companies of crucial investment. The report is equally scathing about the UK’s visa regime, calling it “an absurd act of national self-harm”. Scientists relocating to the UK can face upfront costs exceeding £20,000 for a family of four, that’s 17 times higher than in comparable countries.
To “break the UK out of its doom loop”, the Lords propose creating a National Council for Science, Technology and Growth to coordinate policy and respond quickly to crises affecting the sector. According to the report, the UK’s goal of hosting a trillion-dollar technology company by 2035 risks remaining out of reach unless the government takes bold, coordinated action.
“The Government must stop the bleeding”.
That’s all for this issue! Have a great week and we’ll see you next Monday.

Reply