- Synthesis Spotlight
- Posts
- The Little Base That Could
The Little Base That Could
💡 Solar Storm Pushes Northern Lights Down South

Monday 1st December – Sunday 7th December 2025 | Volume 2, Issue 46 |


Unleashing the Power of Potassium 2-Ethylhexanoate as a Mild and Soluble Base for Pd-Catalyzed C–N Cross-Coupling
W. D. Lambert,† S. Felten,† N. Hadler, N. I. Rinehart, R. Swiatowiec, G. E. Storer, J. Henle, M. A. Servos, C. Yang, A. V. Bay, P. N. Eyimegwu, S. Shekhar* & J. Hartwig*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c07790)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-59c10) 🔓
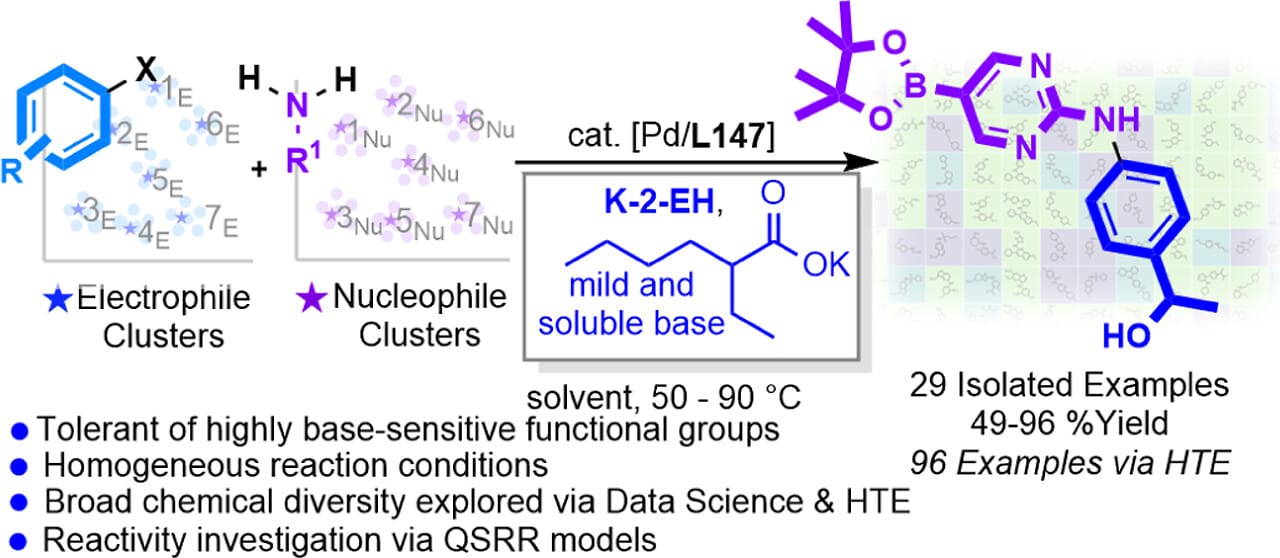
The formation of C–N bonds by Pd-catalyzed cross-coupling typically requires strong or insoluble bases, which limit substrate scope and scalability. Here, the authors report that a combination of a phosphorinane ligand with a soluble carboxylate base, potassium 2-ethylhexanoate (K-2-EH), facilitates the coupling of a range of base-sensitive coupling partners. This combination successfully couples primary aliphatic amines, amides, sulfonamides, heteroaromatic nucleophiles, and acidic secondary nitrogen nucleophiles with various electrophiles. A side-by-side comparison to form selected coupling products in the presence of a range of previously reported bases and ligands showed that the products that decomposed under standard reaction conditions were stable with K-2-EH.
👉️ Write-up from Derek Lowe’s “In the Pipeline”, here.

Iridium(III)-Catalysed Ionic Hydrogenation of Pyridines to Multisubstituted Piperidines
A. Despois & N. Cramer*
Nat. Chem. 2025 (DOI: 10.1038/s41557-025-02008-2)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-78q6b) 🔓
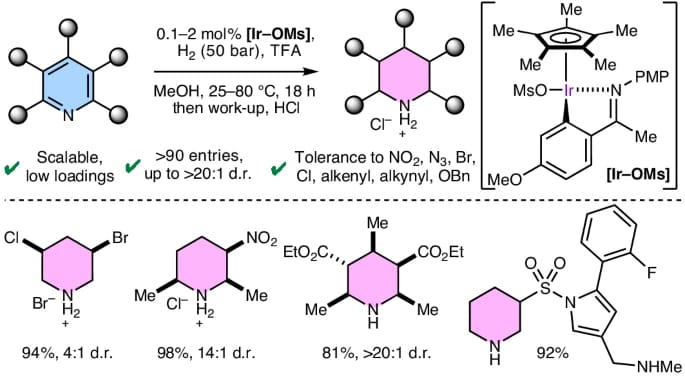
The authors describe a robust and selective iridium(III)-catalysed ionic hydrogenation of pyridines to the corresponding functionalized piperidines. Importantly, highly reduction-sensitive groups—such as nitro, azido, bromo, alkenyl, and alkynyl substituents—are inert, enabling access to a broad range of multisubstituted piperidines in high yields. The method requires low catalyst loadings, is scalable to decagram quantities, and delivers the products as easily isolable and stable piperidinium salts. Applied in a complex late-stage setting, the pyridine motif in several FDA-approved drugs was successfully and selectively hydrogenated.

Carbonylative Ring Expansion of Cyclic Carboxylic Acids
H. Shimono,† M. Kusakabe,† K. Nagao* & H. Ohmiya*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c08640)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-0856k) 🔓

The authors report a synthetic method for the photochemical or electrochemical carbonylative ring expansion of cyclic carboxylic acids. This protocol converts readily available cyclic α-heterocarboxylic acids to lactams, lactones, and thiolactones through incorporation of the exocyclic carbonyl group into the cyclic framework. The reaction is also applicable to 2-aryl-substituted cyclopropane and cyclobutane carboxylic acids. When coupled with α-amino C–H carboxylation of cyclic aliphatic amines, this process functions as a molecular editing platform that enables the carbonylative ring expansion of ticlopidine and nicotine as well as the streamlined synthesis of an ivabradine fragment.
Annulative Skeletal Diversification of Pyrimidines to Expanded Heteroaromatic Space
P. Spieß, A. S. Peloewetse,‡ A. Profyllidou,‡ S. L. Kraus, N. Santarelli, T. Fukuyama, J. T. Kohrt, C. A. Blakemore, C. G. Na, R. Sarpong* & S. W. Bagley*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c14511)
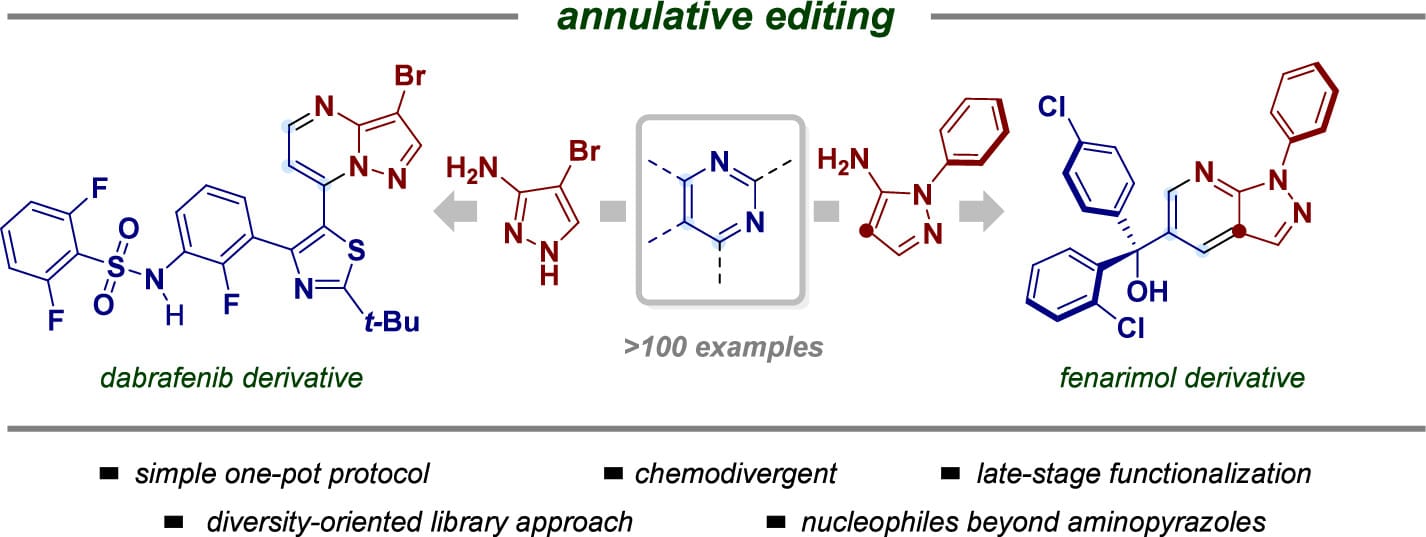
The authors report a two-step, one-pot protocol that converts pyrimidines into 7-azaindazoles and pyrazolo[1,5-a]pyrimidines. Fine-tuning the reaction parameters enables chemodivergent control, allowing selective access to either framework, with the pyrimidine scope including pharmaceutical drug molecules. Moreover, the strategy has been extended to afford differently substituted pyrimidines, partially saturated fused skeletons, and quinoline derivatives.
A Pragmatic Radical Strategy for Dearomative Hydroalkylation of Unactivated Indoles
C. Hu,† R. Li,† R. R. Merchant, Y. Kanda & T. Qin*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c17556)
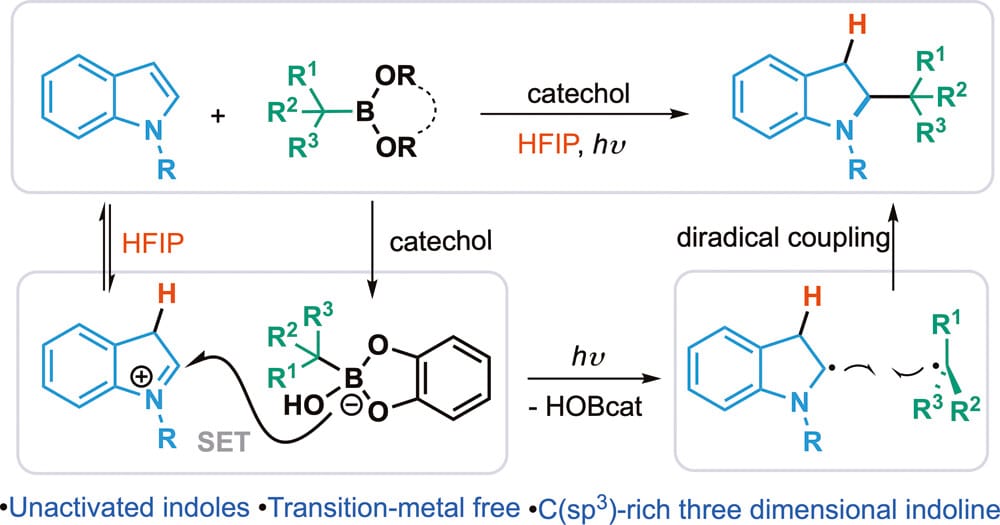
The authors report a metal-free and operationally simple method with broad functional group tolerance for the dearomative hydroalkylation of indoles using alkyl boronates. This strategy advances the radical-based dearomatization strategy to previously unreactive indole substrates, enabling access to a variety range of three-dimensional indolines.
Iridium-Catalyzed Enantioselective C(sp3)–H Borylation of Bicyclo[1.1.0]butanes
T. Jia, L. Chen, W. Sun* & S. Xu*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c16702)
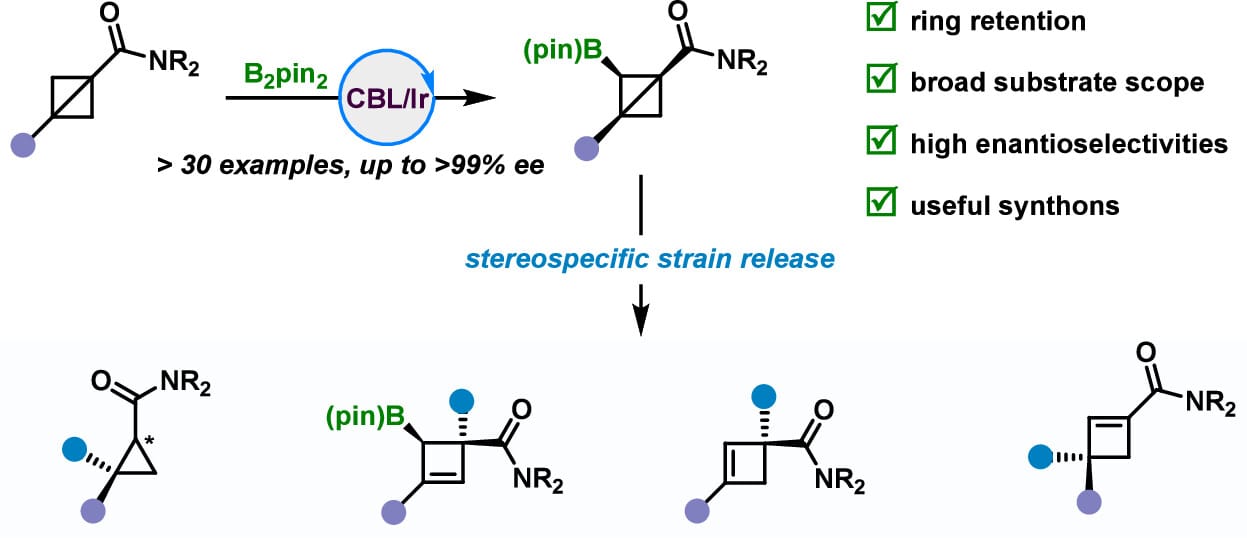
The authors report an iridium-catalyzed enantioselective C(sp3)–H borylation of BCBs, providing access to a range of optically active borylated bicyclo[1.1.0]butanes (BCBs) with high enantioselectivities. Synthetic applications were also demonstrated, particularly in the stereoselective preparation of optically active multisubstituted cyclobutenes.
Total Synthesis and Anticancer Study of (+)-Verticillin A
W. Knauss, X. Wang, M. G. Filbin, J. Qi* & M. Movassaghi*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c16112)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-k6tgk) 🔓
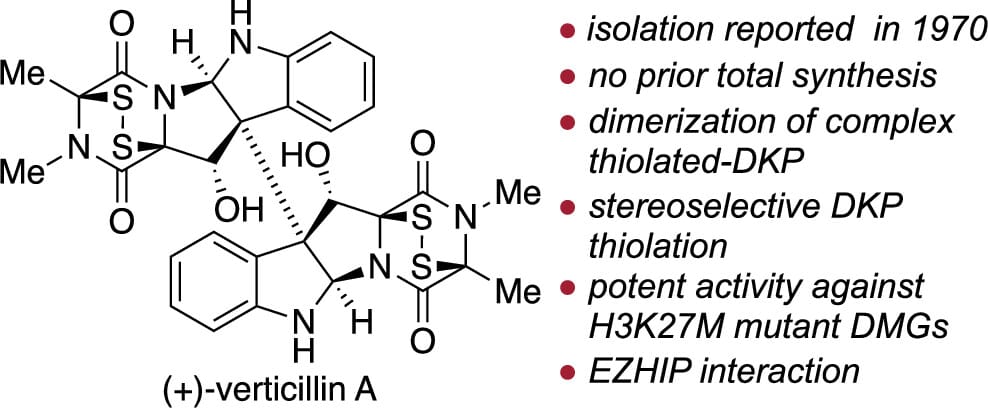
The authors report the first total synthesis of (+)-verticillin A, a fungal metabolite isolated over 50 years ago. Initial attempts at sulfidation of a dimeric diketopiperazine (DKP) resulted in incorrect stereochemistry for the epidithiodiketopiperazine (ETP) substructures. A method was later developed to introduce the disulfide with correct stereochemistry using benzhydryl hydrodisulfide before dimerization. To protect the sensitive ETPs, they masked the disulfide as alkyl sulfides before radical dimerization and photochemical desulfonylation. This process led to the synthesis of (+)-verticillin A, the first dimeric ETP with C12 oxygenation synthesized. (+)-Verticillin A and its derivatives showed potent activity in cancer cell lines, regulating histone lysine 27 trimethylation (H3K27me3) levels and inducing apoptosis.
Unified Total Synthesis of Phrymarolin and Haedoxan Natural Products
J. Paciorek, A. D. F. Guy, A. Sudau, D. M. Barber & T. Magauer*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c16676) 🔓

The authors disclose a unified synthetic route to the furofuran lignans phrymarolin I and II as well as the insecticidal natural products haedoxan A and D. The furofuran core was constructed using a formal [3+2] cycloaddition, followed by a samarium(II) iodide promoted-cyclization. While this sequence enabled the synthesis of phrymarolin I and II in eight steps, attempts to unmask the ortho-quinone necessary for a bioinspired formal [4+2] cycloaddition were unsuccessful, initially preventing access to the haedoxans. Revising the choice of the arene substitution pattern enabled the formation of the requisite ortho-quinone followed by a bioinspired cyclization to the 1,4-benzodioxane motif of the haedoxan framework. Finally, late-stage diversification at the acetal position enabled completion of the synthesis of haedoxan A and D and their analogues in 13 steps.

Electrochemical Azolation of Electron-rich Fluoroarenes: A Controlled Redox Chain Unlocks C–N Bond-forming e-SNAr
B. D. Akana-Schneider, J. S. Genova & J. Derosa*
Angew. Chem. Int. Ed. 2025, Early View (DOI: 10.1002/anie.202520841)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-jl8jt) 🔓
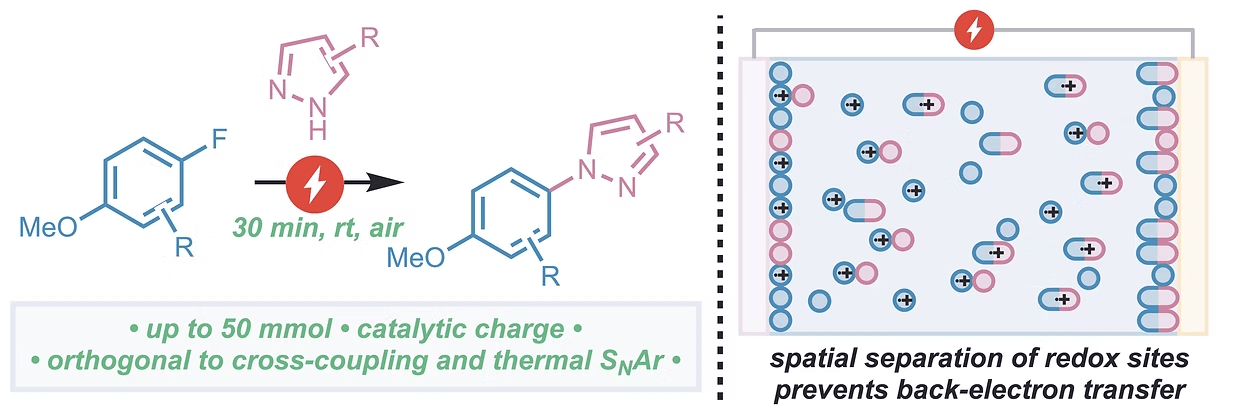
Anodic oxidation unlocks C–N bond formation in electron-rich fluoroarenes. Spatial separation of redox events extends the lifetime of active intermediates, expands the scope of nucleophilic aromatic substitution reactions, and promotes a new mechanism for SNAr reactions: uphill redox catalysis. Powered by voltage control, azolation occurs quickly and selectively; reactions proceed with catalytic charge and can be easily scaled in a batch setup.
Activation of Cyanate Anions by Phosphine Radical Cations Enables Formal Hydrocarbamoylation of Alkenes
P. Vojáčková & A. Studer*
Angew. Chem. Int. Ed. 2025, Early View (DOI: 10.1002/anie.202524782) 🔓
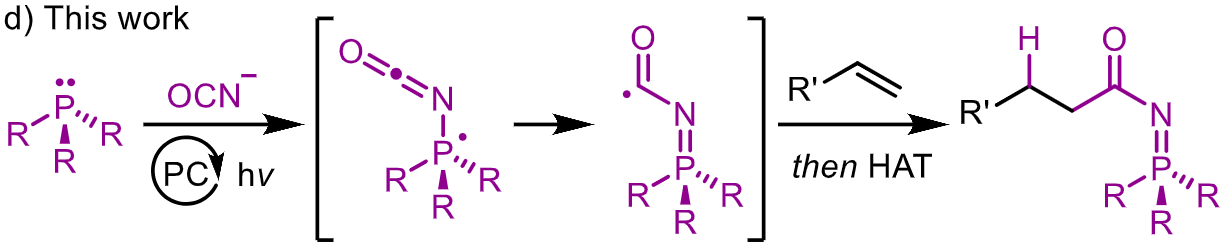
A photocatalytic formal hydrocarbamoylation reaction that employs a cyanate anion as the C1 source and provides N-acyl iminophosphorane products from activated alkenes is described. Mechanistic investigations suggest generation of a phosphoranyl radical by addition of the cyanate anion to a phosphine radical cation, which enables the delivery of the carbamoyl group across alkenes.
18F-Radiopharmaceutical Diversification Enabled by Deaminative Cross-Electrophile Couplings
I. F. Ogilvy, J. Ford, S. Ortalli, E. Renders, T. R. Hayes, S. Liu, I. Mortiers, A. Nikolopoulou, A. M. Sorlin, A. A. Trabanco, M. Tredwell, P. J. J. A. Buijnsters, R. Salter & V. Gouverneur*
Angew. Chem. Int. Ed. 2025, Early View (DOI: 10.1002/anie.202522650) 🔓
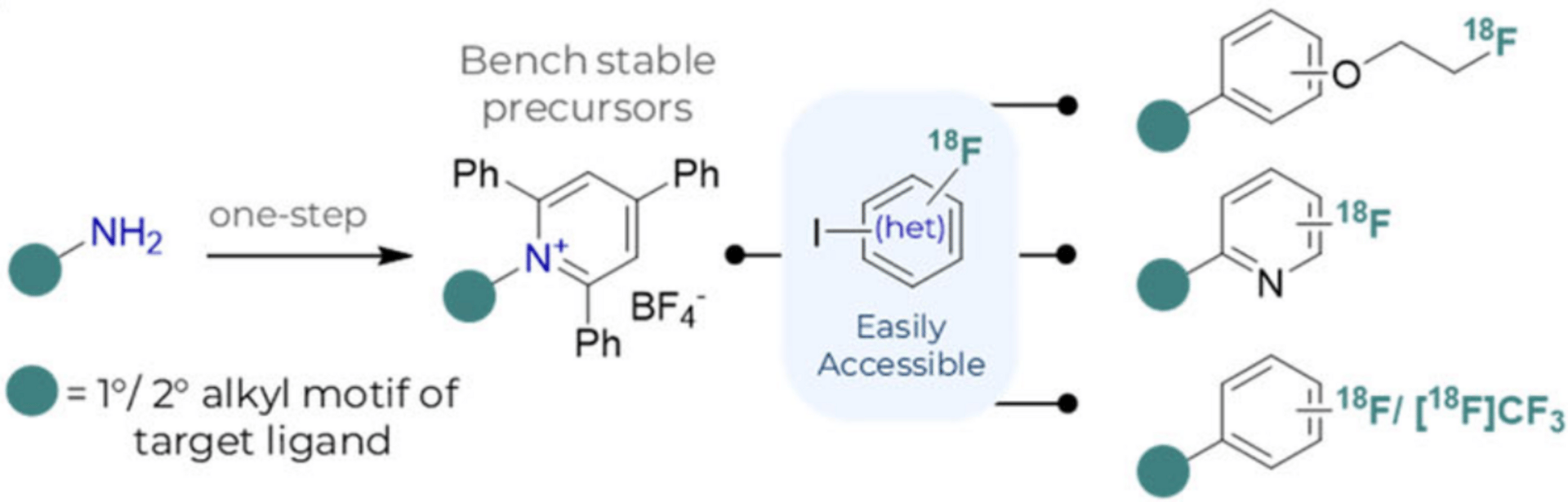
A general Ni-mediated Csp2–Csp3 cross-coupling expedites access to 18F-radiopharmaceuticals, essential for positron emission tomography imaging and drug discovery. This late-stage diversification approach was implemented across three user-friendly automated protocols affording 18F-radiotracers in sufficient quantities for imaging. Tailored high-throughput experimentation rapidly discovers radiochemically applicable reaction conditions.

A C1-Homologative Trifluoromethylation: Light-Driven Decarboxylative Trifluoroethylation of Carboxylic Acids
F. Belnome, A. Pulcinella, S. Bonciolini, M. Lepori, O. P. Datsenko, Z. He, M. Gasparetto, P. K. Mykhailiuk, B. de Bruin* & T. Noël*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-9jfwq) 🔓
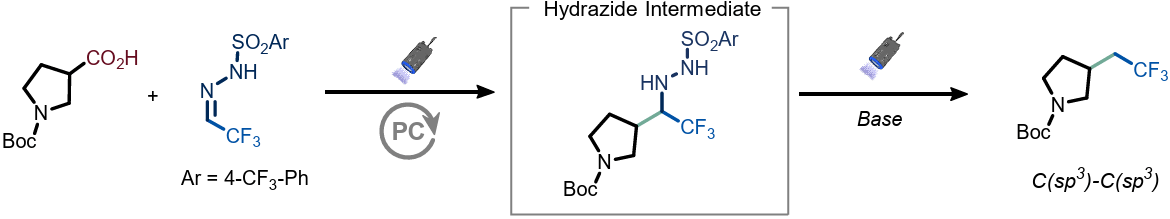
The authors report a general approach for the decarboxylative trifluoroethylation of aliphatic carboxylic acids under visible-light irradiation. The transformation proceeds via photoinduced generation of a carbon-centered radical that adds to a bench-stable sulfonyl hydrazone reagent derived from trifluoroacetaldehyde, followed by light-driven fragmentation to furnish the desired trifluoroethylated products. The reaction operates under mild conditions, exhibits broad substrate scope, including primary, secondary, and tertiary acids, and tolerates diverse functional groups.
Strain-Release Pentafluorosulfanylation of Carbonyl-Containing Disubstituted Bicyclobutanes: Insight on SF5···C=O Interactions and a Fortuitous Path to SF5-Containing Oxa[2.1.1]bicyclohexanes
A. M. Stephens, J. A. Olvera, Y. Kraemer, T. Vu, W.-Y. Kong, S. Chaturvedi, J. M. Wang, D. J. Tantillo* & C. R. Pitts*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-xtbsz) 🔓
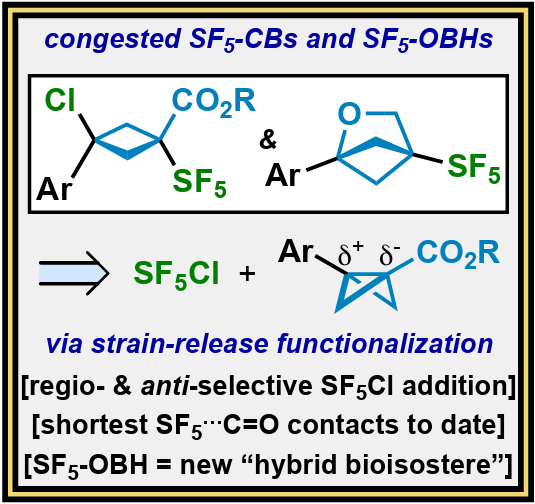
A regio- and diastereoselective method for strain-release pentafluorosulfanylation of 3-aryl-1-carbonyl-substituted BCBs was developed that led to two fortuitous discoveries. The resultant SF5-cyclobutanes (SF5-CBs): (i) act as model systems for studying exceptionally close SF5···C=X noncovalent interactions (X = C, NR, O), and (ii) provide access to a new class of “hybrid bioisostere”: SF5-oxa[2.1.1]bicyclohexanes (SF5-OBHs).
Photosensitizer-Free Infrared-Light-Induced Radical Reactions
A. J. A. Dias, K. Tanaka, M. Uchiyama & Y. Nagashima*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-6xnhk) 🔓
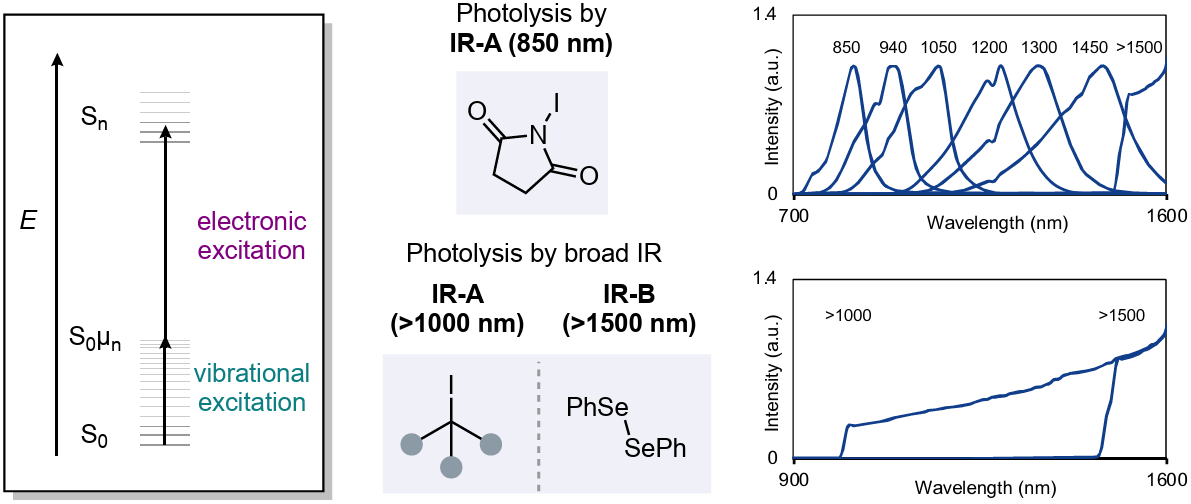
The authors report radical reactions driven by IR-A (~1000 nm) or IR-B light (~1500 nm) via vibration-mediated photolysis in the absence of a photosensitizer. This method promotes diverse transformations, including iodination, alkylation, and selenation reactions, with wider scope, higher selectivity, and deeper penetration through reaction media, compared with those of conventional irradiation with visible light. Applications include irradiation through obstacles, one-pot sequential transformations, and flow chemistry in opaque tubing.

Northern Lights Down South
🔭 Northern Lights Down South. Nature have just released their selection of the best science images in November, featuring an unusually far-reaching aurora that also earned NASA’s Astronomy Picture of the Day on 14th November. Driven by some of the strongest solar activity in decades, the Northern Lights pushed far beyond their typical Arctic range, with colourful displays reported as far south as Spain and across multiple US states.
This photograph, taken by astrophotographer Samil Cabrera at Shired Island, Florida, captures a bright Northern Taurid meteor streaking across the sky, framed by an unexpected wash of auroral glow. While the Taurid meteor shower is a predictable November visitor, auroras at such low latitudes are far more unusual. The dramatic display was fuelled by a series of Earth-directed coronal mass ejections—massive eruptions of plasma and magnetic fields from the Sun—that triggered intense geomagnetic storms and pushed the auroral oval deep toward the equator.
That’s all for this issue! Have a great week and we’ll see you next Monday.

Reply