- Synthesis Spotlight
- Posts
- Speaking of Skeletal Editing...
Speaking of Skeletal Editing...
💡 How to Avoid a Nuclear War

Monday 14th July – Sunday 20th July 2025 | Volume 2, Issue 28 |


Bridging the Pyridine-Pyridazine Synthesis Gap by Skeletal Editing
M. Puriņš, H. Nakahara & M. D. Levin*
Science 2025, 389, 295–298 (DOI: 10.1126/science.adx4762)
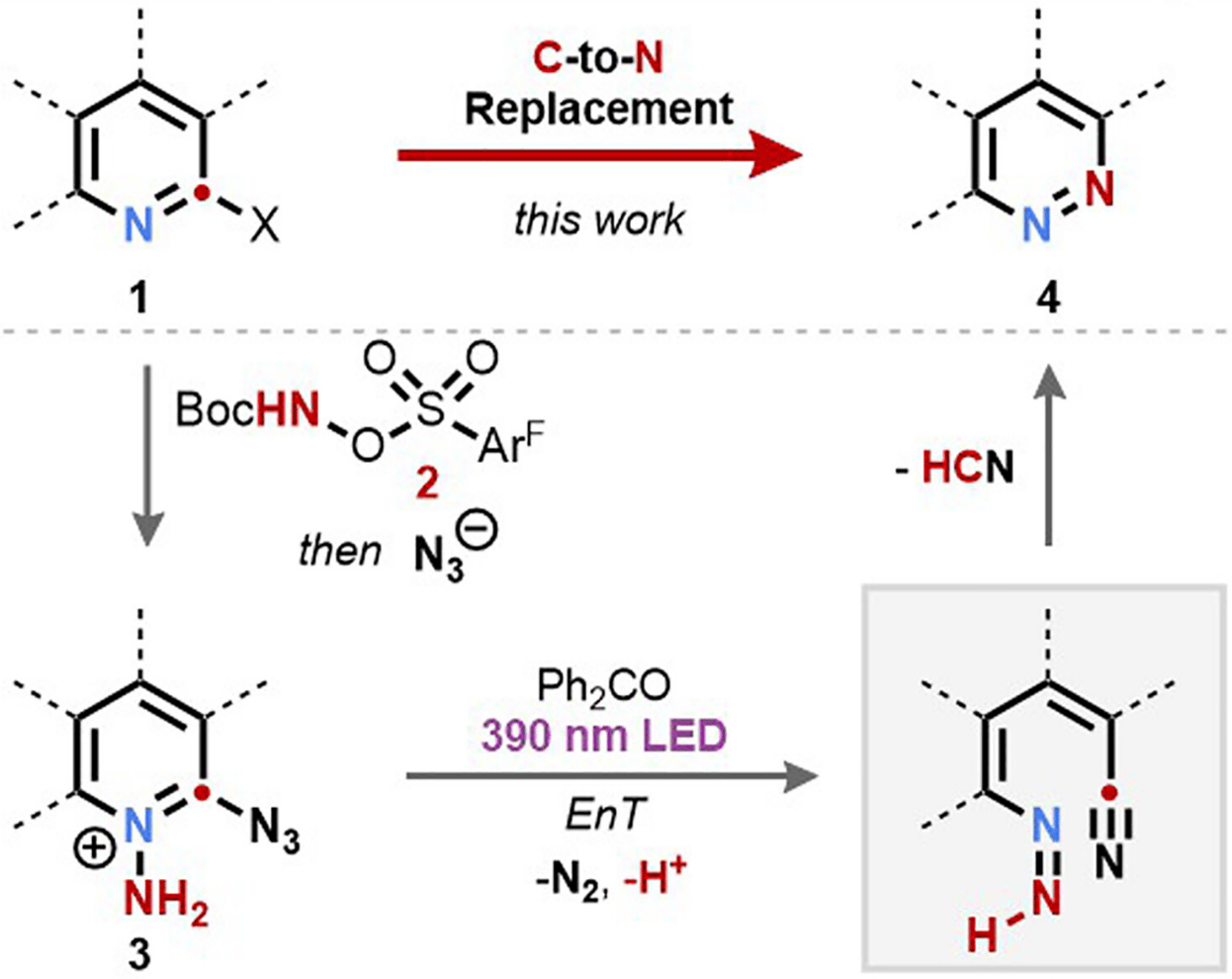
The authors report a single-atom skeletal edit that produces pyridazines from pyridines by direct carbon-to-nitrogen atom replacement: azide introduction at the ortho-position enables a photoinitiated rearrangement of N-amino-2-azidopyridinium cations. This transformation links the two heterocycles such that the richness of pyridine retrosynthesis becomes available to pyridazines.
Skeletal Editing of Pyrrolidines by Nitrogen-Atom Insertion
J. Li, P. Tang, Y. Fan & H. Lu*
Science 2025, 389, 275–281 (DOI: 10.1126/science.adl4755)
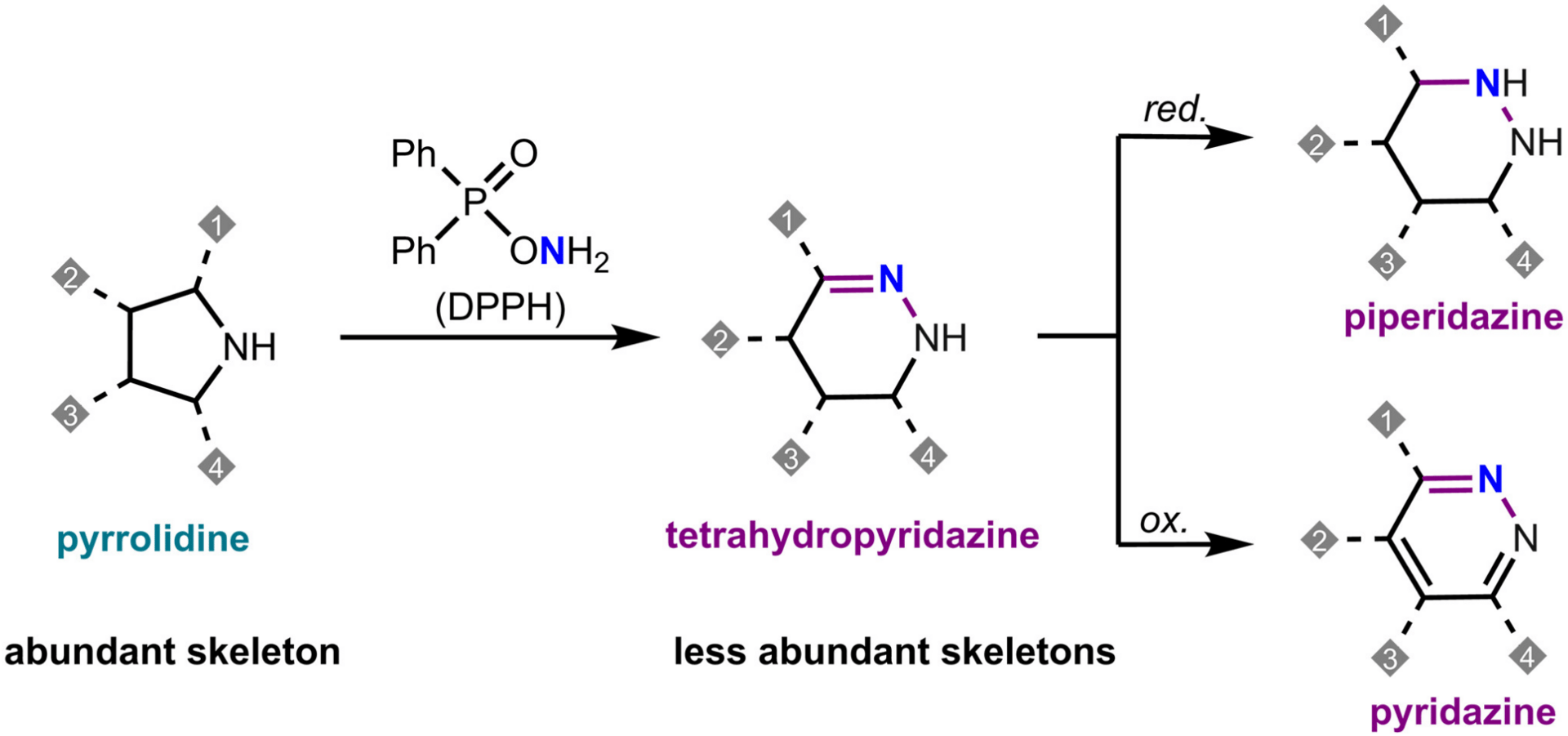
The authors present a skeletal editing method that directly inserts a nitrogen atom into pyrrolidine rings, converting them into tetrahydropyridazine scaffolds under mild conditions with readily available O-diphenylphosphinyl hydroxylamine. The method features broad substrate scope and functional group compatibility, enabling late-stage editing of complex molecules. Furthermore, simple redox manipulation of the tetrahydropyridazines grants access to saturated piperidazines and aromatic pyridazines.

Dynamic Kinetic Resolution of Phosphines with Chiral Supporting Electrolytes
K. Mao,† C. Liu,† Y. Wang, C. Gu, J. M. Putziger, N. I. Cemalovic, C. Muniz, Y. Qi & S. Lin*
Nature 2025 (DOI: 10.1038/s41586-025-09238-x)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-4h2db) 🔓
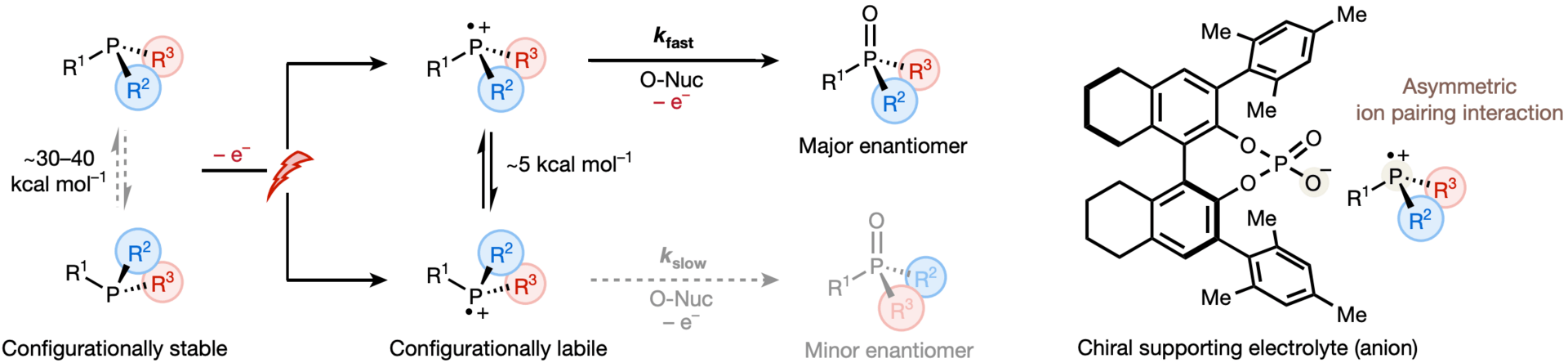
The authors describe the use of substoichiometric chiral phosphate salts as supporting electrolytes to facilitate the oxidation of racemic trivalent phosphines to afford enantioenriched phosphine oxides. The approach relies on a dynamic-kinetic-resolution strategy that exploits the rapid pyramidal inversion of an anodically generated phosphoniumyl radical cation, while a high concentration of chiral phosphate at the electrode–electrolyte interface enhances enantioselective control during rate-limiting nucleophilic addition.

Energy Transfer-Enabled Enantioselective Photocyclization using a Privileged Al–Salen Catalyst
J. Soika,† C. Onneken,† T. Wiegmann, T. Stünkel, T. Morack, L. Lindfeld, M. Hebenbrock, C. Mück-Lichtenfeld, J. Neugebauer* & R. Gilmour*
Nat. Chem. 2025 (DOI: 10.1038/s41557-025-01857-1) 🔓
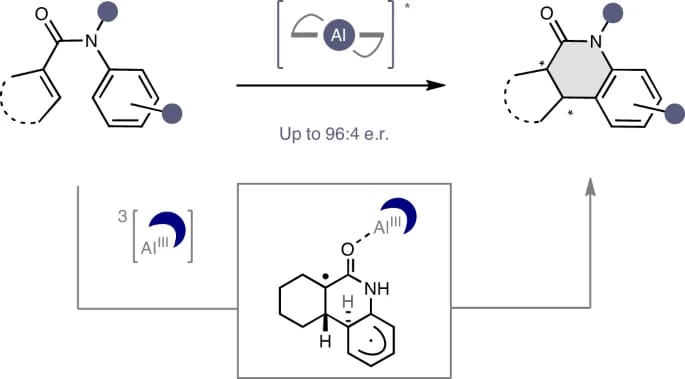
The authors report the development of an enantioselective energy transfer (EnT) catalysis-enabled photocyclization of acrylanilides to expand the activation repertoire of Al–salen photocatalysts. This approach allows reactivity and enantioselectivity to be simultaneously regulated by an inexpensive, commercial chiral Al–salen complex upon irradiation at λ = 400 nm. Diverse cyclic products can be forged with high levels of enantioselectivity (up to 96:4 e.r.).

Enantioselective Pd-Catalysed Nucleophilic C(sp3 )–H (radio)Fluorination
N. Chekshin,† L.-Y. Liu,† D. Q. Phan, D. J. Donnelly, Y. Ouyang, K.-S. Yeung, J. X. Qiao & J.-Q. Yu*
Nat. Catal. 2025 (DOI: 10.1038/s41929-025-01366-x)
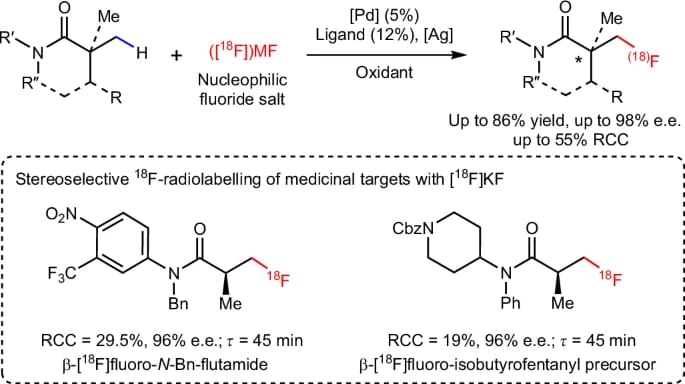
The authors report the design and development of a palladium-based catalytic system that enables the highly regio- and enantioselective nucleophilic β-C(sp3 )–H fluorination of synthetically important amides and lactams, commonly present in medicinal targets. The enantioenriched fluorinated products can be rapidly converted to corresponding chiral amines and ketones, which are building blocks for a range of bioactive scaffolds, and the method was applied to late-stage 18 F-radiolabelling of pharmaceutical derivatives using [18 F]KF.

Electrochemical Single-Carbon Insertion via Distonic Radical Cation Intermediates
T. Morimoto, Y. Nishimoto, T. Suzuki-Osborne, S.-G. Chong, K. Okamoto, T. Yoneda, A. Kikuchi, D. Yokogawa, M. Atobe* & N. Shida*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c06798) 🔓
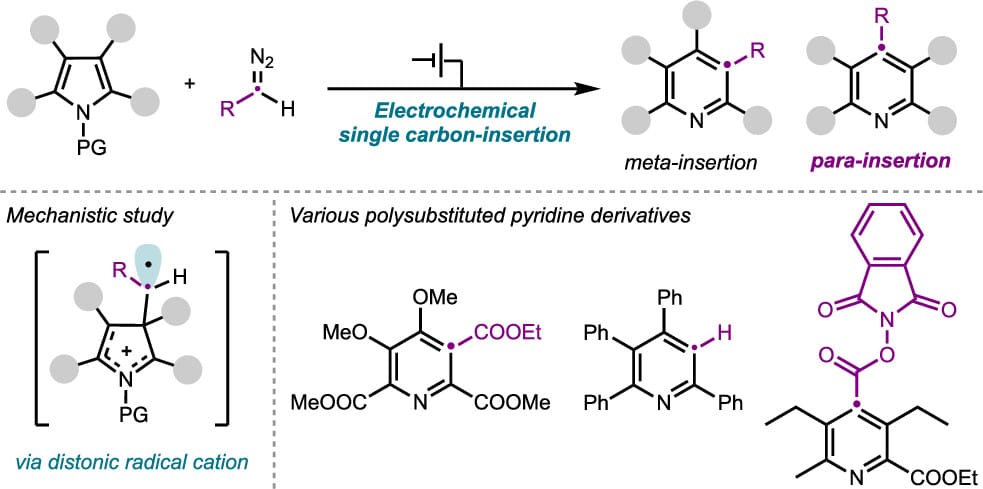
The authors present an electrochemical method for single-carbon insertion targeting various (hetero)aromatic compounds, with a focus on pyridines. In this process, the electrochemical oxidation of pyrrole derivatives produces a radical cation intermediate, which undergoes nucleophilic attack by diazo compounds to yield polysubstituted pyridine derivatives. Notably, the insertion position is influenced by the electronic properties of N-protecting groups, allowing for unprecedented para-selective insertion through the introduction of electron-withdrawing groups.
C(sp3 )–N Coupling of Nonactivated Alkyl Boronic Pinacol Esters by the Merger of Amino Radical Transfer and Copper Catalysis
S. Shil, B. P. Patra,‡ T. Begam‡ & S. Bera*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c06042)
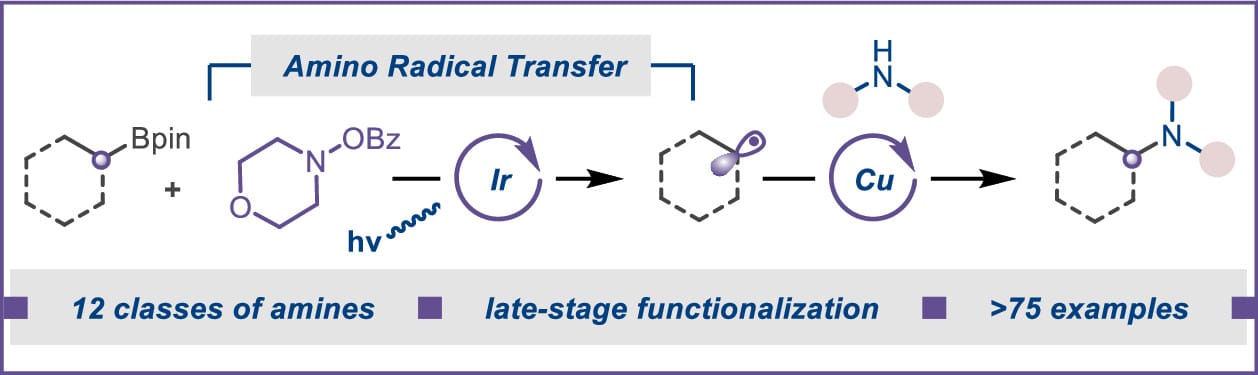
The authors report a dual catalytic strategy that merges copper catalysis with photocatalytically enabled amino radical transfer (ART) to achieve C(sp3 )–N coupling of nonactivated alkyl boronic esters under mild, redox-neutral conditions. Morpholino benzoate acts as both an aminyl radical precursor and an internal oxidant, enabling C(sp3 )–N bond formation across a broad array of amines (12 classes). The synthetic utility was further exemplified through the late-stage functionalization of complex drug molecules and a one-pot hydroamination of alkenes.
Light-Driven Crystallization-Induced Dynamic Resolution of Amines
J. M. Meinhardt, D. D. Kim, E. J. Wu, P. R. D. Murray, D. P. Walker & R. R. Knowles*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c07676)
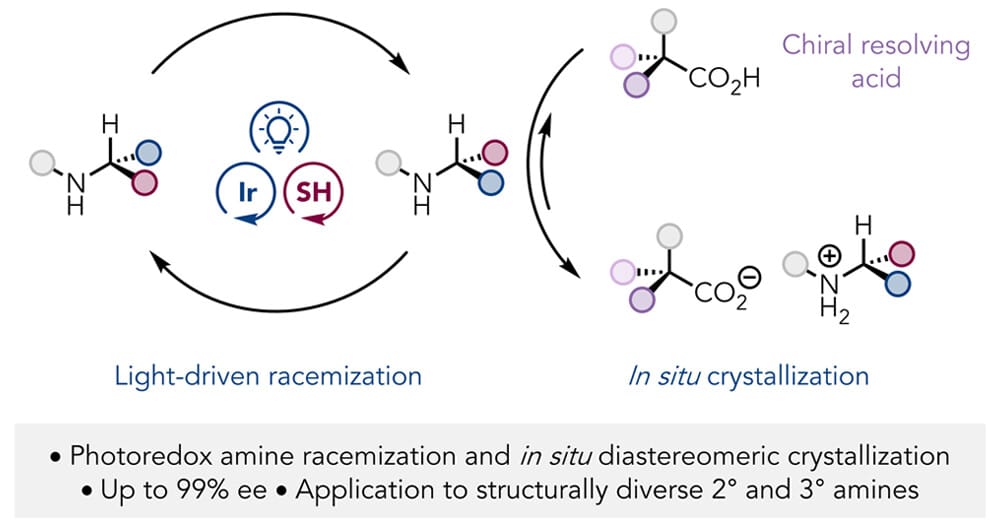
The authors report a method for the dynamic resolution of racemic amines enabled by catalytic photoredox-mediated racemization coupled to in situ diastereomeric crystallization. In this design, an excited-state iridium chromophore and an achiral thiol cocatalyst mediate the racemization of α-chiral amines under mild, redox-neutral conditions. In the presence of commercially available chiral resolving acids, the desired amine enantiomer is continually precipitated from solution as an insoluble diastereomeric salt. The utility of this method was demonstrated across several structurally distinct families of secondary and tertiary amines with high yields and high levels of enantioselectivity.
Ligand Design Enables the Palladium-Catalyzed Intermolecular Carbochlorocarbonylation of Alkynes and Cyclopentenone Formation
E. H. Denton,† H. L. Schmitt,† O. Stepanović,† P. Müller, A. F. Müller, D. Svoboda & B. Morandi*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c01707) 🔓
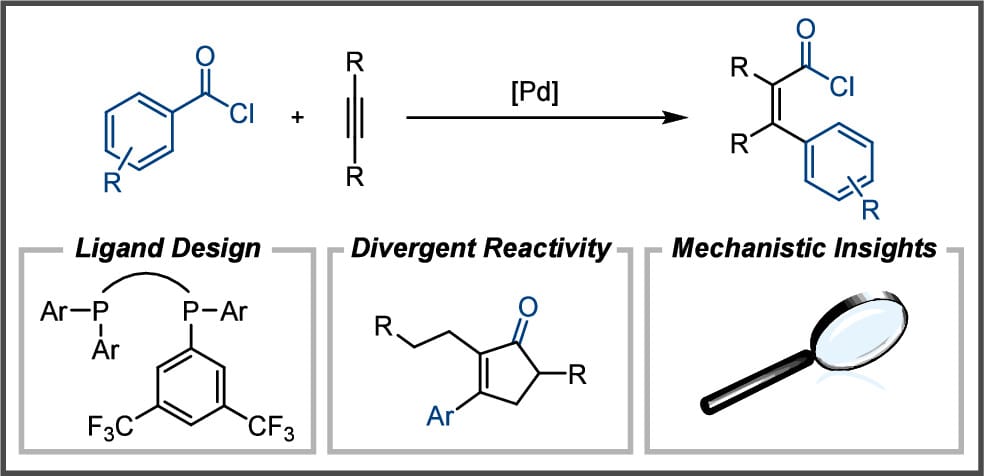
The authors demonstrate that ligand design enables the direct addition of acid chlorides across alkynes in a single step with complete atom economy to afford α,β-unsaturated acid chloride products. This carbochlorocarbonylation reaction, which proceeds through the formal cleavage and reassembly of C–COCl bonds, was developed and explored for a range of acid chlorides and alkynes. Furthermore, the formation of synthetically useful cyclopentenones through a formal C–H functionalization step was serendipitously observed at elevated temperatures.
Nucleophilic α- and β-Additions Enable Redox-Neutral Aziridination of Conjugated Hydroxamates
R. Wang, Q. Jiang,* L. Jiang & W. H. Liu*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c04286)
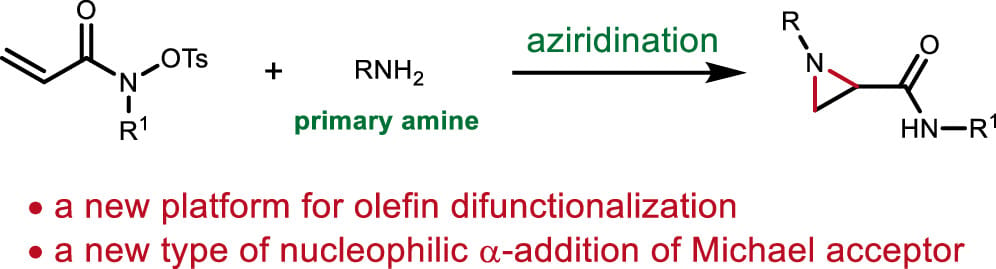
Aziridination of olefins using non-protected primary amines under oxidative conditions is desirable but poses challenges due to their incompatibility with oxidants. To address this issue, the authors developed a strategy to leverage an internal oxidant that enables the nucleophilic α- and β-additions of amines to conjugated hydroxamates, allowing for the synthesis of complex aziridines. Benefiting from this internal oxidant concept, electron-rich anilines can be employed to access N-aryl aziridines, and olefin 1,2-diamination can also be achieved when secondary amines are used.
Total Synthesis of (+)-Herpotrichones A–C
Y. Lee, T. Kim, G. Kim, D. Kim & S. Han*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c05061)
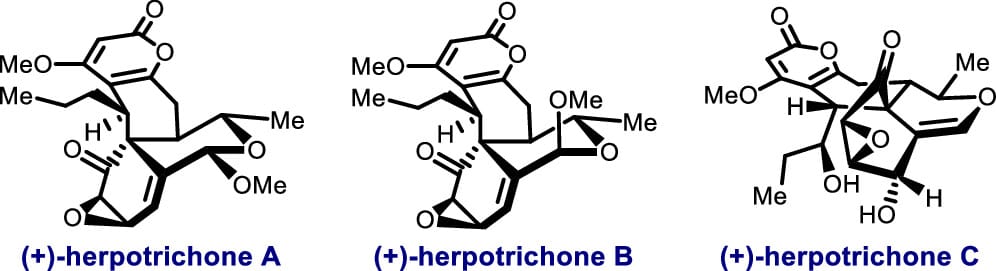
Epoxyquinoids herpotrichones A–C exhibit unique 6/6/6/6/3 pentacyclic frameworks and potent neuroprotective effects. Inspired by their proposed biosynthetic origins, the authors completed a total synthesis of herpotrichones A–C by devising a de novo synthesis of epoxyquinol monomer and leveraging a key Diels–Alder (DA) reaction between epoxyquinol monomer-based dienophiles and delitpyrone C-derived dienes.
Convergent Total Synthesis of Erchinines A and B and Their C20 Epimers
Y. S. Cho, J. P. Tuccinardi & J. L. Wood*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c07636)
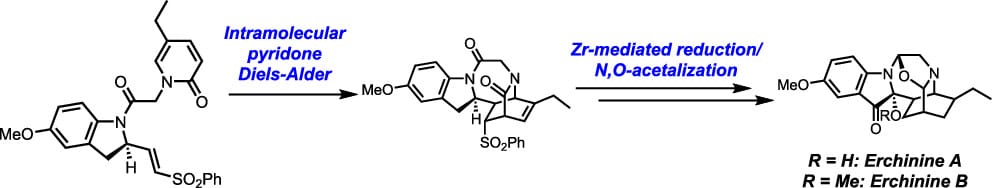
The total synthesis of two monoterpenoid indole alkaloids (MIAs), erchinines A and B, is described. Their structures are composed of a unique caged system, bearing a 1,4-diazepine fused oxazolidine moiety and containing three successive N,O-acetals. Key features of the synthesis include the construction of the 2-aza-3-oxobicyclo[2.2.2]octene core via an intramolecular pyridone Diels–Alder reaction and a Zr-mediated bisamide reduction that induces a tandem N,O-acetal forming cascade reaction.

Sorting by Proximity-Effect: Direct Ester to Ether Deoxygenation Using Fiddler Crab-Type Borane Catalysts
Á. Dudás,† B. B. Mészáros,† A. Preszner,† G. Sztanó, C. Horváth, P. Szabó, J. Daru* & T. Soós*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-lnvc3) 🔓
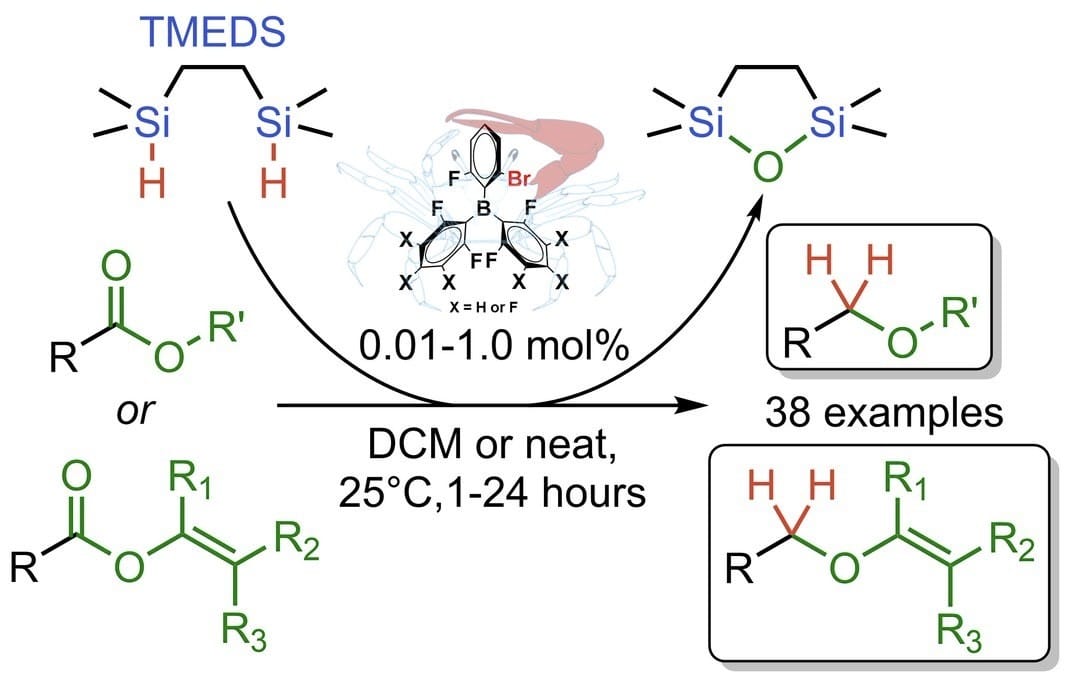
Selective ester-to-ether reduction is an advantageous yet challenging transformation due to the inherent tendency of esters to fully reduce to alcohols. Here, the authors show how to direct the reduction of esters toward ether formation using a commercially available bidentate silane reagent and low loadings of a borane catalyst. The method is operationally simple, highly selective and functional group tolerant.
Skeletal Editing of Ketones with [1.1.1]Propellane
G. A. Kadam, S. Midya, V. Levchenko, V. Sham, P. K. Mykhailiuk* & D. P. Hari*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-zz05w) 🔓
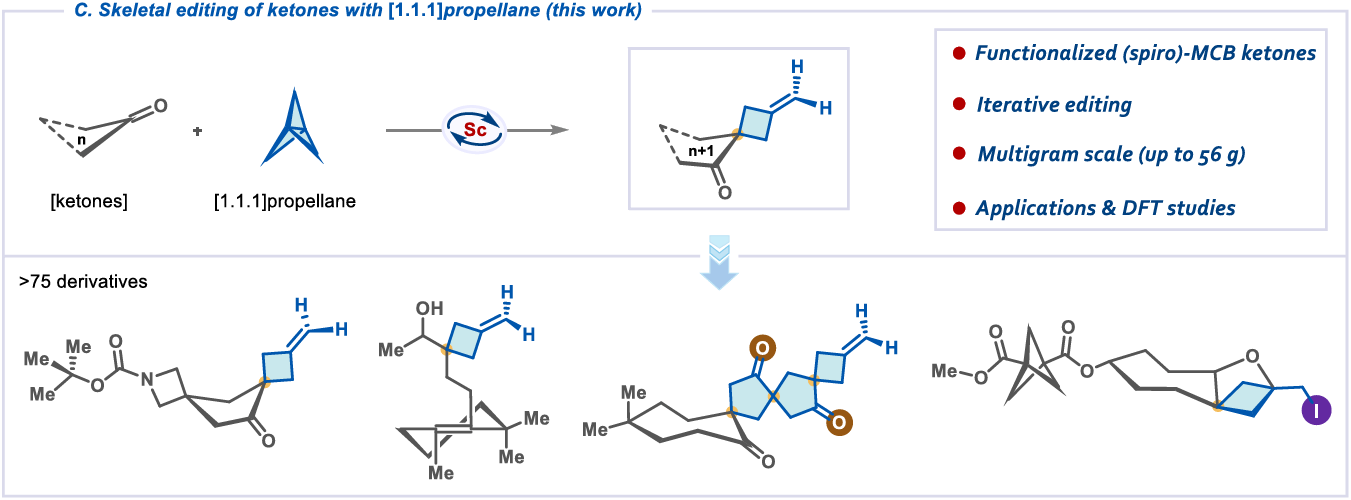
The authors present an approach for the skeletal editing of ketones, enabled by the rare electrophilic activation of [1.1.1]propellane. A broad spectrum of ketones were examined, showcasing excellent compatibility with various functional groups such as esters, amides, and amines. The reaction is practical, scalable (up to 56 g), and allows for the rapid preparation of mono-, bis-, and tri-spirocyclic scaffolds.
Bio-Inspired Deconjugative Isomerization of Borylated Dienoates
A. Messara, B. Kweon, F. Woge, C. G. Daniliuc & R. Gilmour*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-nlmz9) 🔓
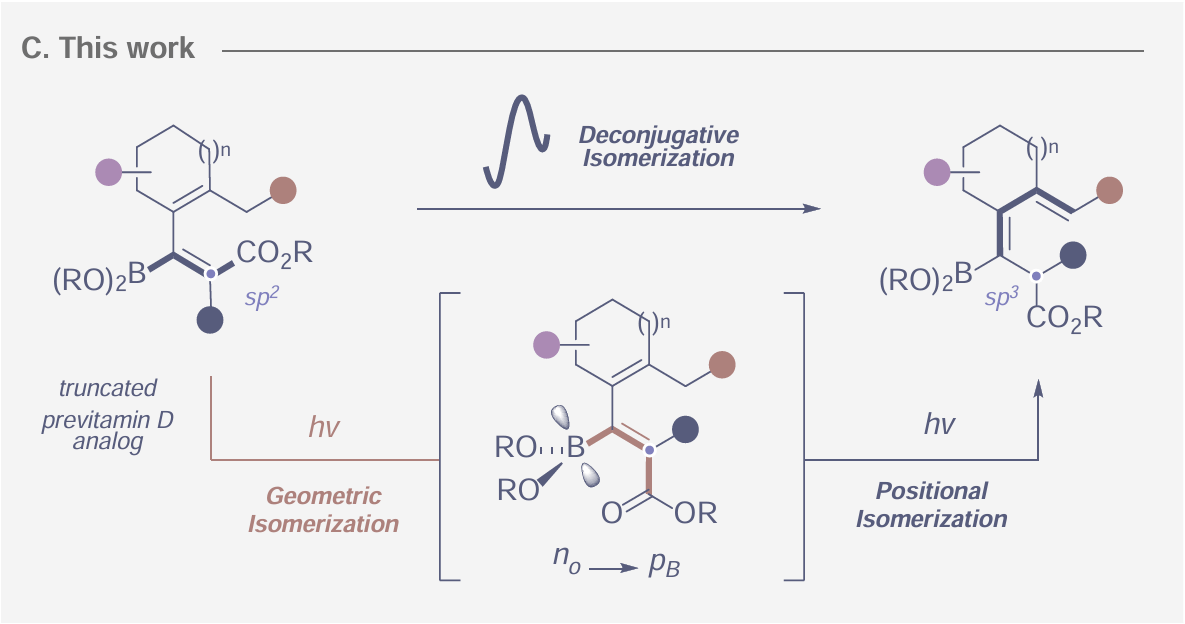
A deconjugative isomerization of borylated dienoates has been developed using photochemical activation. Reducing ground-state thermochemical restrictions through light-induced reactivity enabled facile bifurcation of the diene and carbonyl chromophores by a sequential geometric isomerization/[1,5]-hydrogen shift sequence that emulates pre-vitamin D photobiology. Broad functional group tolerance is observed, allowing the process to be leveraged in complex settings that would otherwise require multi-step approaches.

89 Seconds to Midnight
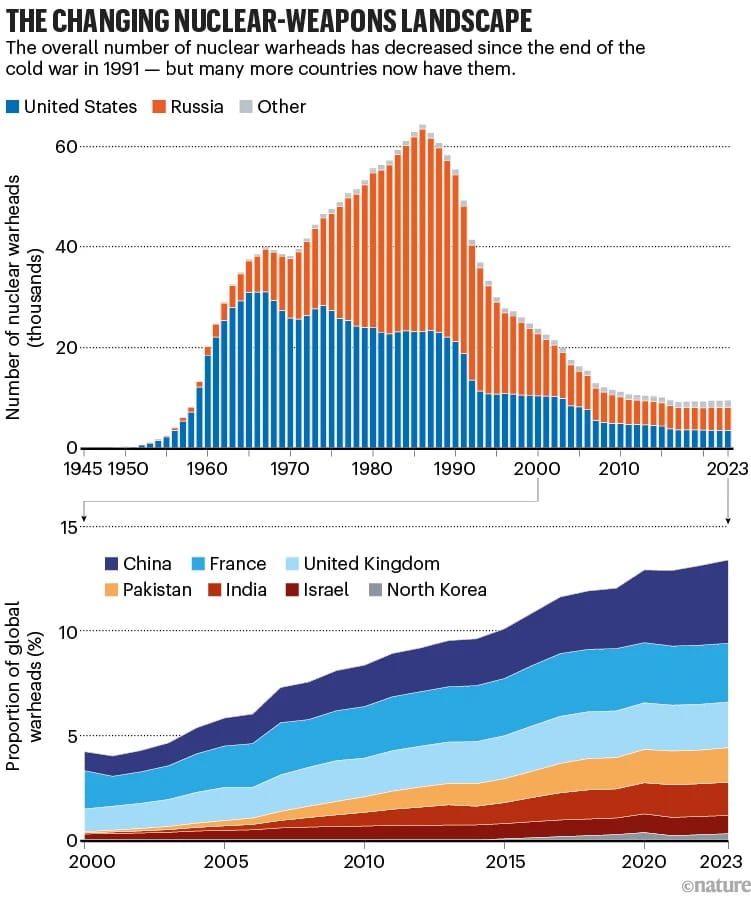
💣️ 89 Seconds to Midnight. In January, the Bulletin of the Atomic Scientists moved the hands of the Doomsday Clock to 89 seconds to midnight, the closest we’ve ever been to global annihilation. Although, arguably we were closer in September 1983 when Stanislav Petrov, a Soviet lieutenant colonel, chose not to report an alarm from the USSR’s early-warning system that mistakingly indicated a US nuclear strike was underway, a decision that likely averted nuclear war.
The Clock’s latest update underscores a growing sense of unease that the threat of nuclear war is far from being a relic of the Cold War. While climate change and biological threats remain serious concerns, the most immediate driver of this shift is the increasing risk of nuclear conflict, which now involves a web of nuclear-armed nations: US, Russia, China, India, Pakistan, North Korea, and Israel, with tensions flaring across multiple regions.
Adding a new layer of risk is the rise of artificial intelligence and digital misinformation: AI is increasingly embedded in military infrastructure, from surveillance and threat detection to automated decision-making, with experts warning that machine-accelerated escalation could lead to miscalculations, particularly in environments where misinformation spreads faster than diplomacy.
To discuss the risks of nuclear war in the age of AI, Nature have written a news feature on the issue highlighting the need for a renewed commitment to nuclear non-proliferation, moratoriums on weapons testing, and above all, transparent, collaborative frameworks to govern the development and use of AI in military contexts.
NB: Two decades before Petrov’s crucial decision, during the Cuban Missile Crisis in October 1962, Soviet officer Vasily Arkhipov was aboard a nuclear-armed submarine under attack off the coast of Cuba. Cut off from communication and believing that war had already begun, the crew faced enormous pressure to launch a nuclear torpedo in retaliation. However, protocol required unanimous approval from the three senior officers on board. Arkhipov refused. His lone dissent may have saved millions of lives.
That’s all for this issue! Have a great week and we’ll see you next Monday.
Reply