- Synthesis Spotlight
- Posts
- Single-Atom Swap: From Indoles to Benzimidazoles
Single-Atom Swap: From Indoles to Benzimidazoles
💡 New World in the Deep: 40+ Species Discovered off Argentina’s Coast

Monday 1st September – Sunday 7th September 2025 | Volume 2, Issue 35 |


Carbon-to-Nitrogen Atom Swap Enables Direct Access to Benzimidazoles from Drug-like Indoles
A.-S. K. Paschke, Y. Brägger, B. B. Botlik, E. Staudinger, O. Green & B. Morandi*
Nat. Chem. 2025 (DOI: 10.1038/s41557-025-01904-x) 🔓

The authors report a method for the carbon-to-nitrogen atom swap in N-alkyl indoles, allowing the direct conversion of indoles to the corresponding benzimidazoles. The reaction leverages the innate reactivity of the indole scaffold to engage in an initial oxidative cleavage step, followed by oxidative amidation, Hofmann-type rearrangement and cyclization. The reaction tolerates a wide range of functional groups, which is demonstrated by the interconversion of 15 drug-like molecules.

Head–Tail Carboboration of Multisubstituted Alkenes Enabled by Chain Recognition
W. Kong,† D. Wu,† H. Wei,† B. Gao, P. Li, Y. Li, Y. Li* & G. Yin*
Nat. Chem. 2025 (DOI: 10.1038/s41557-025-01903-y)

The authors report a head–tail carboboration of multisubstituted alkenes with exceptional site-selectivity, enabled by ligand steric exclusion in a nickel-catalysed chain-walking system. This catalytic system enables selective migratory transformations of tertiary alkyl–metal intermediates across a range of thermodynamically and kinetically accessible reaction site combinations.

Spin-Density-Controlled Radical Coupling for meta-Selective C–H Halogenation of Pyridines
J. Zhang, G. Wu, Y. Zhao, X. Liu, H. Zhu, A. Studer, X. Qi* & Q. Cheng*
Nat. Synth. 2025 (DOI: 10.1038/s44160-025-00869-6)
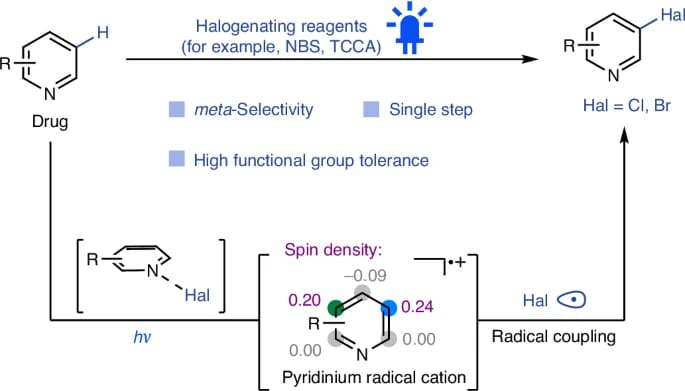
The authors report a visible light-induced single-step meta-selective radical bromination and chlorination of pyridines using commercial reagents. The key intermediates—pyridinium radical cations, with their tendency to undergo radical coupling—were shown to overcome the reluctant halogenation reactivity of pyridines, which differs conceptually from previous umpolung temporary dearomatization strategies.

A Modular Synthesis of Azetidines from Reactive Triplet Imine Intermediates using an Intermolecular Aza Paternò–Büchi Reaction
B. A. Williams, M. J. Tilby, N. A. Parker, M. R. Uehling, J. C. Hethcox, D. Kalyani & M. C. Willis*
Nat. Catal. 2025 (DOI: 10.1038/s41929-025-01405-7) 🔓

The authors show that simple acyclic imines bearing N-sulfamoyl fluoride substituents generate reactive triplet imines that react with a broad range of alkenes to produce azetidine products in high yields. Mechanistic and computational studies confirm the key role of the sulfamoyl fluoride unit in dictating the [2+2] pathway. In addition, the sulfamoyl fluoride substituents offer a convenient reaction site for product functionalization or for traceless removal.

Triplet Carbene Insertion Enables Modular Access to C2-Substituted Bicyclo[1.1.1]pentanes
J.-T. Che,† H.-B. Zhang,† W.-Y. Ding, S.-H. Xiang & B. Tan*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c10649)

The authors report a modular strategy for the direct synthesis of C2-substituted BCPs from readily available bicyclo[1.1.0]butanes (BCBs) and diazo compounds. Leveraging homolytic cleavage of the central bond in BCBs, a triplet energy transfer-initiated carbene insertion process occurs, to form 1,4-biradical species, which are ultimately converted to the target BCPs via rapid radical recombination. The practicability of this process has been demonstrated through the collective replacement of the phenyl moiety with bioisosteric BCP in 15 bioactive molecules.
Divergent Synthesis of 1-Azabicyclo[n.1.1]alkane Bioisosteres via Photoinduced Palladium Catalysis
Y. Zhang, K.-D. Li, S. Yu, K. Pan, H. Xu & H.-M. Huang*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c09500) 🔓

The authors report a switchable radical approach for the synthesis of 1-azabicyclo[2.1.1]hexanes and 1-azabicyclo[4.1.1]octenes through the coupling of azabicyclo[1.1.0]butanes with 1,3-dienes, mediated by a visible-light-driven palladium photocatalytic system. This method exhibits a broad substrate scope, excellent functional group compatibility and has been employed successfully in DNA-encoded library synthesis.
Regioselective Hydroamidation of α,β-Unsaturated Esters Enabled by Lewis Acid/Iron Relay Catalysis
H. Choi, D. Kim & S. Chang*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c08838)

The authors report a dual boron/iron catalytic system that enables the unprecedented hydroamidation of α,β-unsaturated esters to exclusively access α-amidated esters under mild conditions. The strategy harnesses the Lewis acidity of B(C6F5)3 to rapidly generate reactive silyl ketene acetal intermediates, which are subsequently intercepted by in situ generated iron nitrenoids. This protocol is operationally simple, requiring no tailored ligands, light, or electrochemical setup, and proceeds efficiently with only 1 mol% of boron and iron catalysts.
Water as an Electron Donor for Cross-Electrophile Coupling Reactions
J.-L. Li,† C.-M. Zhu,† H.-G. Li, P.-F. Yuan & Q.-Y. Meng*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c12176)

The authors report a divided electrochemical synthesis-based cross-coupling platform in which H2O is oxidized at the anode surface to generate electrons that produce a lower oxidation state nickel catalyst on the cathode surface, enabling XEC reactions without the need for metallic or organic reagents. A wide array of primary and secondary bromides, as well as pharmaceutically relevant (hetero)aryl halides, were identified as viable coupling partners.

Regioselective Electrochemical Borylation of Oxygenated Allylic Electrophiles: Method Development and Synthetic Applications
W.-C. C. Lee,† P.-L. Lagueux-Tremblay,† Z. Jia & S. Lin*
ACS Cent. Sci. 2025, ASAP (DOI: 10.1021/acscentsci.5c01074) 🔓
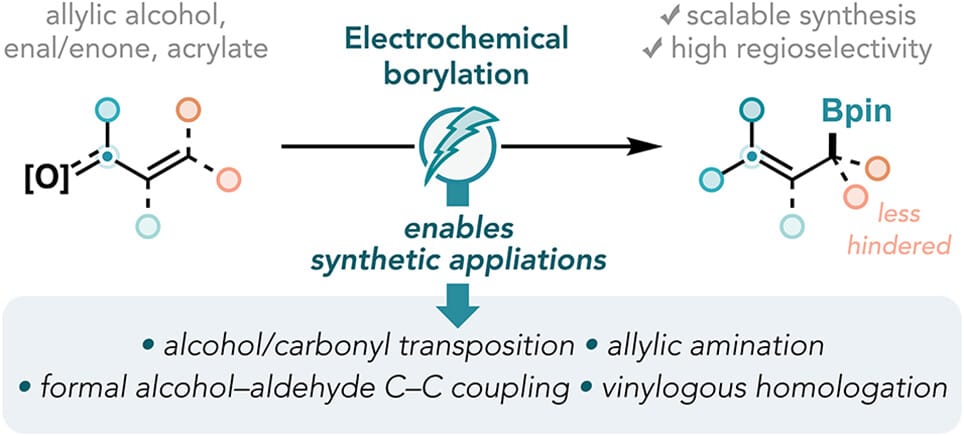
The authors report a general and scalable strategy for the regioselective deoxygenative borylation of allylic alcohols, enals, enones, and acrylates, upgrading these abundant functional groups in feedstock chemicals and natural products into value-added borylated synthetic handles. The utility of this approach was demonstrated in a series of telescoped synthetic sequences, enabling alcohol and carbonyl transposition, formal cross-coupling of alcohols and aldehydes, allylic amination, and vinylogous homologation.

A Broadly Applicable Strategy to Aminate Azines Enabled by Electronically Tuned Phosphine Reagents
R.-R. Liu, J. N. Levy & A. McNally*
Angew. Chem. Int. Ed. 2025, Early View (DOI: 10.1002/anie.202512024) 🔓

The authors describe a strategy for aminating pyridines and other azines via phosphonium salt intermediates. Precisely tuning the electronic properties of the phosphonium ion was key for C–N bond formation via an SNAr-halogenation, SNAr-amination sequence. The process couples a wide range of amine classes with pyridines and is viable for applications such as late-stage amination of complex pharmaceuticals and fragment–fragment coupling reactions.

Terpenoid Synthesis via Convergent Radical Annulation
A. L. Rerick, G. L. Barnes, F. Schneider, L. Oxenfart & P. S. Baran*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-cb46q) 🔓

The development of a convergent radical annulation strategy for the synthesis of complex terpenoids from sclareolide is disclosed. This approach employs a 1,3-diradical synthon to enable rapid C-ring annulation through inter- and intramolecular radical couplings, exemplified in the concise syntheses of serratene and cyclodammarane scaffolds from a common intermediate. Key features include a rapid alternating polarity (rAP) Kolbe electrolysis for onoceradiene assembly, a Co-electrocatalytic MHAT 7-endo-trig cycloisomerization to form the serratene core, and a tandem Fe-mediated reductive olefin coupling/enolate alkylation cascade to forge the [4.3.1] propellane motif of cyclodammaranes with complete diastereocontrol over three contiguous quaternary centers.
Skeletal Editing of Furans into Pyridines
P. Kafle,† S. Yasuda,† D. Nilson, D. Herndon, R. C. Fox & I. Sharma*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-vplrf) 🔓

The authors report a skeletal editing strategy for furans in which sulfenylcarbenes selectively cleave the furan core to generate enone intermediates with a built-in leaving group, which subsequently react with a nitrogen source to restore aromaticity and furnish pyridines. This metal-free approach offers broad functional group tolerance and a conceptually distinct platform for controlled heterocycle remodeling.
Do Amino-Oxetanes Resemble Amides? A Matched Molecular Pairs Property and Structural Comparison
H. Ishikura, C. S. Begg, J. J. Rojas, L. Blagojevic, G. J. Smith, J. Luk, R. A. Croft, C. Romain, C. Choi & J. A. Bull*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-hkhlq) 🔓

The authors present a matched molecular pair study of twelve 3-aryl-3-amino-oxetane and benzamide matched molecular pairs to assess their viability as isosteric replacements. Across the surveyed physicochemical properties (pH stability, solubility, lipophilicity, clearance, permeability), amino-oxetanes exhibited comparable profiles to their amide counterparts, while displaying improved solubility. However, analysis of crystal structures of the amino-oxetane and benzamide pairs highlights the conformational difference and alternative exit vectors available through introduction of the oxetane ring. Amino-oxetanes have significantly lower barriers to rotation, allowing for the potential to flexibly accommodate binding sites of interest. However, the preferred gauche conformation makes the torsion angle and exit vectors of amino-oxetanes more similar to sulfonamides, and on this basis amino-oxetanes provide better like-for-like topological replacements for sulfonamides rather than of amides.
Room-Temperature Decarboxylative Amination of (Hetero)Aromatic Carboxylic Acids
R. Basnet, K. Golian, J. Sampson & J. M. Hoover*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-0jbfd) 🔓

The authors report a room-temperature decarboxylative amination to generate a wide array of primary (hetero)aromatic amines mediated by N-fluorobenzesulfonimide (NFSI). The broad functional group tolerance of this reaction enables efficient amination of biologically relevant small molecules. Mechanistic studies suggest that NFSI provides access to an activated sulfonimide intermediate, a functional equivalent to acyl azide intermediates, while avoiding the hazards associated with organoazides.
Kinetic, Spectroscopic, and Computational Investigation of Oxidative Aminative Alkene Cleavage Reveals an N-Iodonium-Iminoiodinane Pathway
F. Ruepp, V. Grebennikov, M. Avramenko, M.-O. Ebert & B. Morandi*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-6cdgb) 🔓

The authors present an extensive mechanistic study of a recently published oxidative aminative cleavage of alkenes to obtain insights into the under-studied aspects of hypervalent iodine-mediated nitrogen atom insertion. Through this, it was found that the formation of an N-iodonium-iminoiodinane was rate-determining in this reaction and this species is highly electrophilic and capable of concerted, asynchronous transfer of a [PhI–N]+ unit to double bonds. These findings point towards the N-iodonium-iminoiodinane, not an iodonitrene, being the active N-atom transfer agent generated from the combination of hypervalent iodine(III) oxidants and ammonia.
Mild Catalytic Generation of CO2•− via Photolysis of CO2 Carbamate for Hydrocarboxylation Reactions
E. Azzi, M. Rodríguez-Martínez, S. R. N. Kolusu, J. Scarfiello, J. A. Varela & M. Nappi*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-p13zk) 🔓

The authors present a new catalytic activation mode to generate CO2•− directly from CO2 under mild reaction conditions. Key to this methodology is the formation of a CO2 carbamate with a phenothiazine catalyst, which sets the required trigonal geometry for the release of CO2•− via photolysis upon absorption of visible light. The polarity-reversed CO2•− is employed in hydrocarboxylation reactions of alkenes and heterocycles.

Approach to Heterospirocycles for Medicinal Chemistry
C. Rodríguez-Arias,† R. Miguélez,† Y. Holota, P. K. Mykhailiuk* & P. Barrio*
Org. Lett. 2025, ASAP (DOI: 10.1021/acs.orglett.5c03125) 🔓

The gold(I)-catalyzed cycloisomerization of aliphatic 1-bromoalkynes has been applied to the synthesis of heterospirocycles. The reactivity of the C(sp2 )–Br bond in the products allowed for further derivatization of the obtained scaffolds. In this way, spiroheterocycles decorated with a plethora of functional groups, e.g. CO2H, NH2, OH, and Bpin, were readily obtained.

An Underwater Oasis
🌊 An Underwater Oasis. Nature have just released their selection of the best science images in August, featuring a video of an extraordinary deep-sea world teeming with fascinating creatures. Using a remotely operated vehicle, scientists ventured into the Mar del Plata Submarine Canyon, about 300 kilometres off the Argentine coast. At depths of around 3,500 meters, the canyon is shaped by the meeting of two powerful currents—one warm and salty, the other cold and nutrient-rich—creating a thriving ecosystem. The expedition has already uncovered more than 40 species believed to be new to science.
In personal news, my (now) wife, Gillian—who is also an organic chemist—and I were married on Friday! We’ll be on our honeymoon at the end of the month so the last issue in September will be Monday 22nd September before taking two weeks off to return on Monday 13th October, covering any papers that were missed over that period. |  |
That’s all for this issue! Have a great week and we’ll see you next Monday.
Reply