- Synthesis Spotlight
- Posts
- Radicals, Biocatalysis, and C–H Functionalisation. Saxitoxin, Simplified.
Radicals, Biocatalysis, and C–H Functionalisation. Saxitoxin, Simplified.
💡 Let There Be Light: Succulents Engineered to Glow from Within

Monday 25th August – Sunday 31st August 2025 | Volume 2, Issue 34 |


Scalable Total Synthesis of Saxitoxin and Related Natural Products
Y. Guo,† Y. Li,† S. Chen, Y. Wu, O. Poll, Z. Ren, Z. Liu, R. Vlkolinsky, M. Bajo, C. K. Prier, K.-J. Xiao, B. F. Cravatt, M. Roberto* & P. S. Baran*
Nature 2025 (DOI: 10.1038/s41586-025-09551-5)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-qj8f5) 🔓

Saxitoxin (STX), a potent neurotoxin from shellfish, offers immense pharmaceutical potential due to its interaction with voltage-gated sodium channels. Hundreds of synthetic studies towards this end have been disclosed thus far, yet, a fully modular and scalable approach to the family remains elusive. Here, the authors show how a tactical combination of radical retrosynthesis, biocatalysis, and C–H functionalization logic can be combined to solve this problem resulting in a scalable approach to the STX family in less than 10 steps including the first total synthesis of neosaxitoxin (neoSTX), a hydroxylated naturally occurring STX analog previously under clinical investigation. The modular synthesis enables access to diverse analogs, that were previously inaccessible, and now have been evaluated through electrophysiological assays for biological activity.

Unlocking Azole Chemical Space via Modular and Regioselective N-Alkylation
C. Dorval, A. D. Matthews, K. Targos, S. N. Alektiar, D. E. Holst, Z. Tan, M. Muuronen, J. B. Diccianni, J. E. Gómez, K. M. Sanders, I. A. Guzei & Z. K. Wickens*
Nat. Chem. 2025 (DOI: 10.1038/s41557-025-01891-z)

The authors introduce an approach to prepare a broad array of important but difficult-to-access N-alkyl azole compounds through the introduction of a base-catalysed hydroazolation of alkenylthianthrenium electrophiles. This strategy circumvents the classical challenge of azole alkylation regiocontrol through an unusual reversible C–N-bond-forming step that exploits the thermodynamic differences between azole N-alkylation isomers. The reaction furnishes a class of versatile azolothianthrenium building blocks that provides a general platform to investigate diverse N-alkyl azole molecules.

Site-Selective Ru-Catalysed Saturation of Unactivated Arenes via Directed 6π Activation
C. Yu, L. Yiu, Z. Zhang & G. Dong*
Nat. Catal. 2025 (DOI: 10.1038/s41929-025-01404-8)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2024-kt0lk) 🔓

The authors report a Ru-catalysed directed arene saturation, which selectively reduces the aryl group adjacent to the directing moiety. Remarkably, a number of easily reducible functional groups are compatible with the mild reaction conditions. The synthetic utility of this method is demonstrated in the streamlined synthesis of cis-atovaquone, gram-scale reactions and late-stage saturation of complex bioactive compounds.

Total Synthesis of (−)-Psathyrin A Enabled by Radical Cyclization
W. Zhao, R. Al-Ahmad & M. Dai*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c11534) 🔓

The authors describe the total synthesis of (−)-psathyrin A, an antibacterial diterpene possessing a unique 6/4/5/5 tetracyclic carbon skeleton and seven contiguous stereocenters, including three adjacent all-carbon quaternary centers. The sole six-membered ring was introduced through a Suzuki–Miyaura cross-coupling, and a gold(I)-catalyzed Conia-ene reaction constructed the 5/5-fused bicyclic ring system. Following Birch reduction of the aromatic ring, hydrolysis, and double bond isomerization, a Baran reductive olefin coupling set the key four-membered ring. This radical cyclization completed the tetracyclic carbon framework for subsequent peripheral decorations, achieving the first total synthesis of (−)-psathyrin A in 19 steps.
Photoinduced, Copper-Catalyzed Enantioconvergent Azidation of Alkyl Halides
F. Zhong, R. L. Anderson, P. H. Oyala & G. C. Fu*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c10003)

The authors report that a photoinduced chiral copper catalyst (generated in situ from commercially available components) can achieve enantioconvergent azidations of racemic secondary and tertiary alkyl halides (α-halocarbonyl compounds). The resulting enantioenriched alkyl azides are useful end points and building blocks in synthesis.
Diversity-Oriented Method Development: Multifunctional Homoallylic Amines and Azabicyclooctanes Bearing a Bridgehead Enamine
Z. C. Mueller,† R. P. Ponzi,† X. Z. Sui, P. Liu* & A. H. Hoveyda*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c09730)
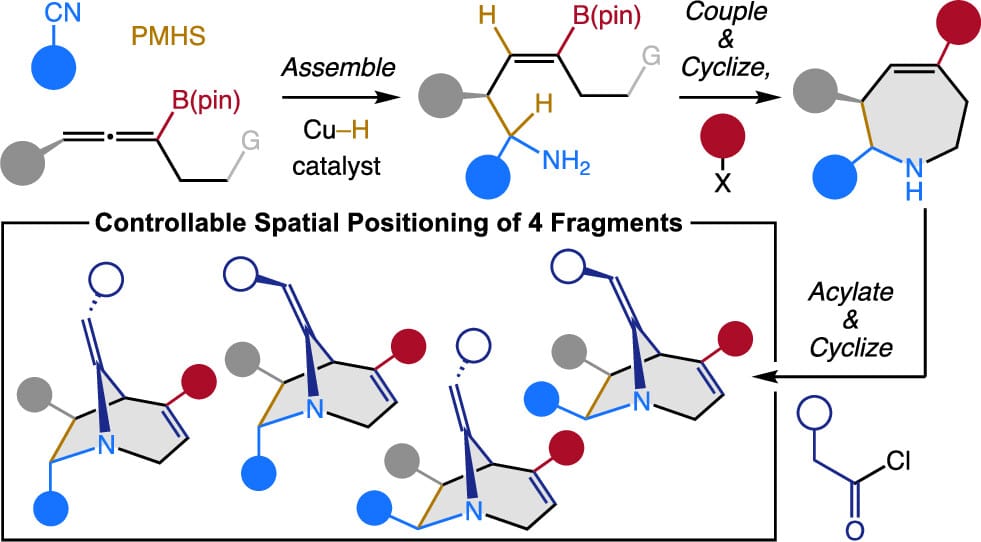
Two methods, designed primarily for applications in diversity-oriented synthesis, are introduced: The first is a Cu–H-catalyzed multicomponent process that involves a nitrile and a trisubstituted allenyl boronate to produce homoallylic α-secondary NH2-amines, containing a trisubstituted alkenyl boronate, formed with >98% enantiospecificity, in up to >98:2 d.r. and as a single alkene isomer (>98% Z). The second method is a diastereoselective triflic anhydride-mediated conversion of an unsaturated azapane-amide, generated from the first products, to azabicyclooctanes (ABCOs) that contain a bridged enamine moiety and a trisubstituted alkene, which can be functionalized chemo- and stereoselectively.
Enantioselective Radical–Radical Cross-Couplings of β-Hydroxy Amides and N-Hydroxyphthalimide Esters via Ni/Photoredox Catalysis
L.-L. Zhang, L.-J. Li, N. Wang, H. Yu & Z.-P. Yang*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c10963)

The authors report a platform that combines photoredox and chiral nickel catalysis to tame transient primary and secondary alkyl radicals under mild conditions. A one-pot variant is also presented wherein the N-hydroxyphthalimide (NHP) ester is generated in situ, enabling the streamlined synthesis of enantioenriched products in a single step from commercially available carboxylic acids and easily accessible alcohols. The utility of this method is demonstrated by diverse transformations to valuable scaffolds and bioactive molecules.
Synthesis of Highly Substituted Alkenes from Terminal Alkynes
B. W. Gardner, C. P. Chung, M. R. Pu & G. Lalic*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c11173)

The authors present a new approach for synthesizing tri- and tetrasubstituted alkenes through the catalytic coupling of terminal alkynes with alkylboranes and organohalides. The reaction enables the efficient and precise generation of multiple isomers of tri- and tetrasubstituted alkenes, and the coupling of alkylboranes with terminal alkynes in the presence of a proton source was demonstrated, which provides complementary stereoselectivity in the synthesis of trisubstituted alkenes to broaden access to various isomers of highly substituted alkenes.
C(sp3 )H/N(sp2 ) Cross-Coupling Reaction for the Synthesis of Tertiary Arylamines via Fluxional SOX·Pd(II) Catalysis
B. G. Budaitis, Z. M. Firestein, A. C. Yue & M. C. White*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c08883)

The authors report a palladium(II) [Pd(II)]/sulfoxide-oxazoline(SOX)/phosphoric acid-mediated C(sp3 )H/N(sp2 ) cross-coupling of arylamine nucleophiles and terminal olefins to furnish >80 diverse tertiary (3°) arylamines in excellent yields (average 82%) and selectivities (>20:1 E/Z, >20:1 linear/branched). The generality of the reaction enabled the synthesis of six pharmaceuticals and derivatives (e.g., zafirlukast) and five late-stage drug fragment couplings (e.g., flutamide-duloxetine).
Divergent Total Syntheses of Elisapterane and Relevant Diterpenoids Assisted by In Silico Structure Reassignment
C.-H. Liu,† H. Gong,† Y. Sheng, W. Wang, Q. Xia* & H. Ding*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c11010)

The authors describe their synthetic endeavors toward elisapterane and relevant diterpenoids through a bioinspired divergent strategy based on late-stage D-ring formation logic. The synthesis of the misassigned structure of elisapterosin F led the authors to revisit the structure elucidation of these natural products. Application of NMR calculation-based in silico structure reassignment disclosed several unheeded structural mutations for elisapterane diterpenoids and revised the structures of elisapterosins A, D, and F. With a computation-rerouted synthetic blueprint, the collective total syntheses of elisapterosins A–F was accomplished, alongside biogenetically related aberrarone, elisabanolide, and 3-epi-elisabanolide.
Asymmetric Total Synthesis of (−)-Psychotriadine Featuring [3,3]-Rearrangement and Stereo-Controllable Ring-Contraction
Q. Yang, H. Ouyang, H. Yu, F. Li & T. Xu*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c11779)
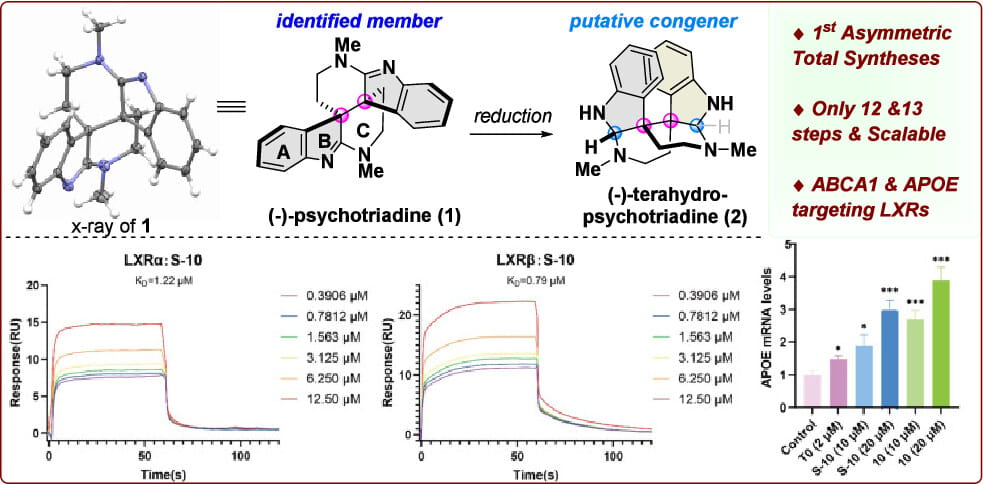
The first asymmetric total syntheses of (−)-psychotriadine and its putative congener tetrahydropsychotriadine were accomplished in 12 and 13 steps, respectively, featuring Meerwein–Eschenmoser–Claisen rearrangement and Fischer indolization/Plancher rearrangement cascade. A stereochemically controllable dearomative ring contraction was utilized to create the vicinal quaternary stereocenters. Biological studies revealed that natural product precursor S-10 acts as an agonist toward liver X receptors (LXRs, KD = 1.22/0.79 μM), which upregulates protein levels of ABCA1 and APOE, exhibiting potential as a drug lead in developing medicine for Alzheimer’s disease.

Iridium-Catalyzed Reductive Deoxygenation of Esters for the Synthesis of Sterically Hindered Ethers
Y. A. Almehmadi, A. J. Passmore, P. Gabriel & D. J. Dixon
Angew. Chem. Int. Ed. 2025, Early View (DOI: 10.1002/anie.202508301) 🔓
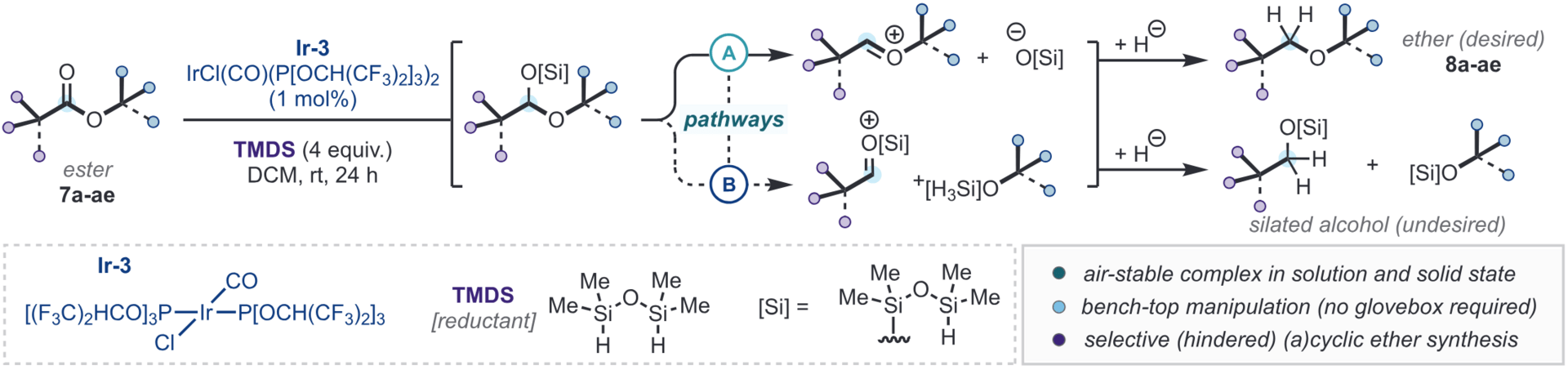
A general method for accessing sterically hindered ethers via reductive deoxygenation of esters is reported. By employing commercially available and bench-stable IrCl(CO)(P[OCH(CF3)2]3)2 (1–2 mol%) and tetramethyldisiloxane (TMDS) as reductant, this single-vessel approach operates under mild conditions and provides a range of alkyl, aryl, and benzyl ethers—both acyclic and cyclic—in good to excellent yields.

Pyridine-to-Pyridazine Skeletal Editing
W. Choi,† A. Jang† & S. Hong*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-1jc74) 🔓
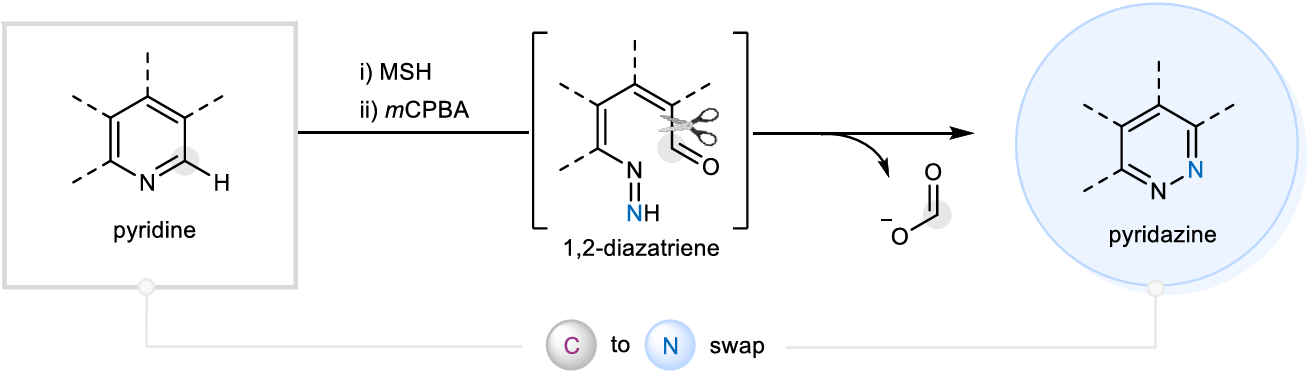
The authors report a skeletal editing strategy that converts pyridines into pyridazines through N-amine assembly then m-chloroperoxybenzoic acid (m-CPBA) mediated ring-remodeling via a 1,2-diazatriene intermediate to effect carbon to nitrogen substitution. This two-step process is operationally simple, runs at ambient temperature in air, and requires no UV irradiation or preinstalled groups. The method shows broad functional-group tolerance, including complex, drug-derived molecules.

Photocatalytic Hydrogenation of Alkenes Using Ammonia-Borane
E. Rivera-Chao, W. J. Olivier, M. J. Tilby* & D. Leonori*
Chem 2025, Online Now (DOI: 10.1016/j.chempr.2025.102711) 🔓
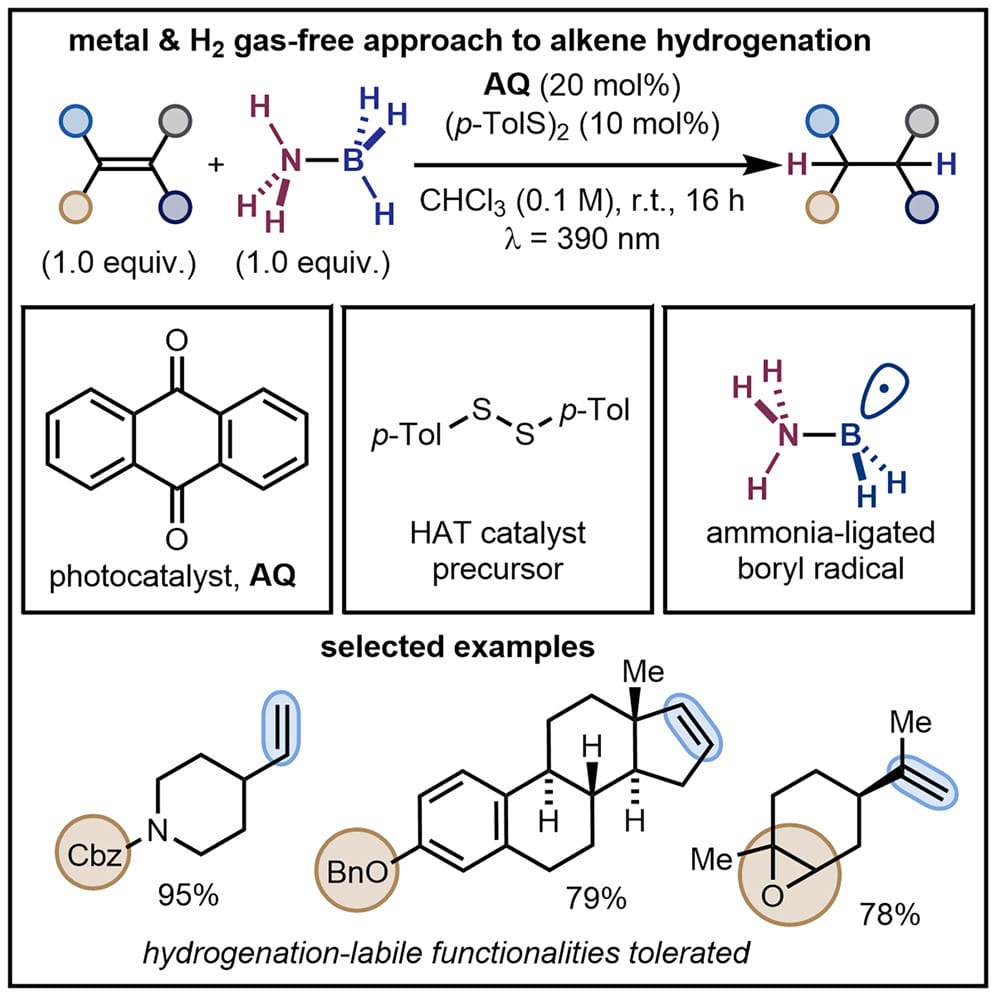
The authors introduce a photocatalytic strategy for alkene hydrogenation using H3N-BH3. The system harnesses visible light with a diaryl ketone photocatalyst to convert H3N-BH3 into its corresponding boryl radical. This open-shell species undergoes a complex sequence of transformations, including a key halogen-atom transfer with the solvent, in situ generation of alkyl borane intermediates, H-atom transfer to generate an aminyl radical, and subsequent β-fragmentation to engage with a thiol co-catalyst. This H2- and metal-free approach enables the transformation of a broad range of alkenes, tolerating functional groups typically incompatible with standard protocols.
Radical Sorting as a General Framework for Deaminative C(sp3 )–C(sp2 ) Cross-Coupling
D. Chattapadhyay, E.-C. Liu,‡ M. J. Diaz,‡ A. Maity, B. A. Bratten & Q. Michaudel*
Chem 2025, Online Now (DOI: 10.1016/j.chempr.2025.102716)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-j3lq0) 🔓

The authors present a general method for deaminative cross-coupling relying on a dual-catalytic system that generates geminate pairs of non-identical alkyl radicals via photosensitization of unsymmetrical 1,2-dialkyldiazenes, then selectively engages the desired radical species in C(sp3 )–C(sp2 ) bond formation. This Ni-mediated “radical sorting” of geminate radical pairs is key in obtaining high yields and avoiding side products. The approach enables the functionalization of a broad array of structurally diverse primary amines—including peptide derivatives and synthetic pharmaceutical intermediates.

From Mono-N-Protected Amino Acids to Pyridones: A Decade of Evolution of Bifunctional Ligands for Pd(II)-Catalyzed C–H Activation
Y.-H. Li,† Y. Ouyang,† J.-L. Yan, N. Chekshin & J.-Q. Yu*
Acc. Chem. Res. 2025, ASAP (DOI: 10.1021/acs.accounts.5c00503)

In this Account, the authors summarize their efforts in the rational design of different classes of pyridone-based ligands for Pd(II)-catalyzed diverse C–H functionalization. Emphasis is placed on site-selective and enantioselective functionalization at methylene and remote C–H positions, and how ligand architecture dictates both reactivity and enantioselectivity. Special attention is given to the use of bidentate pyridone ligands in enabling transformations of synthetically valuable native substrates.

Living Colour
Scientists crafted glow-in-the-dark #succulents that recharge in sunlight. Injected with light-emitting phosphor, the plants can shine as bright as a small night light. cell.com/matter/fulltex…
#SCAU Shuting Liu & colleagues
@Matter_CP— Cell Press (@CellPressNews)
3:00 PM • Aug 27, 2025
🌵 Living colour. Researchers at South China Agricultural University have produced bright luminescent plants by injecting succulents with micron-sized strontium aluminate phosphors, creating plants that emit light equivalent to a small nightlight. The work, published in Matter, represents the first demonstration of multicolour luminescence in houseplants and using this method, the team generated luminescence in green, blue-violet, red, and white, with emission persisting for up to ~10 minutes before recharging with white light was required.
The succulent, Echeveria “Mebina”, was particularly effective but each leaf required direct injection, taking ~10 minutes per plant, but the phosphor-treated succulents remained rechargeable and stable across a 10-day experimental period. However, questions remain regarding long-term plant health and potential toxicity of the particle-laden tissue. Nevertheless, the work demonstrates a practical, low-cost route to multicolour plant luminescence, expanding possibilities for some pretty unique decorative applications.
That’s all for this issue! Have a great week and we’ll see you next Monday.
Reply