- Synthesis Spotlight
- Posts
- Lighting The Way: Enantioselective Photoredox Catalysis
Lighting The Way: Enantioselective Photoredox Catalysis
💡 From Boom to Bust: Is There an AI Bubble?

Monday 17th November – Sunday 23rd November 2025 | Volume 2, Issue 44 |


An Anion-Binding Approach to Enantioselective Photoredox Catalysis
P. Vojáčková & E. N. Jacobsen*
Science 2025, 390, 831–836 (DOI: 10.1126/science.adz3362)
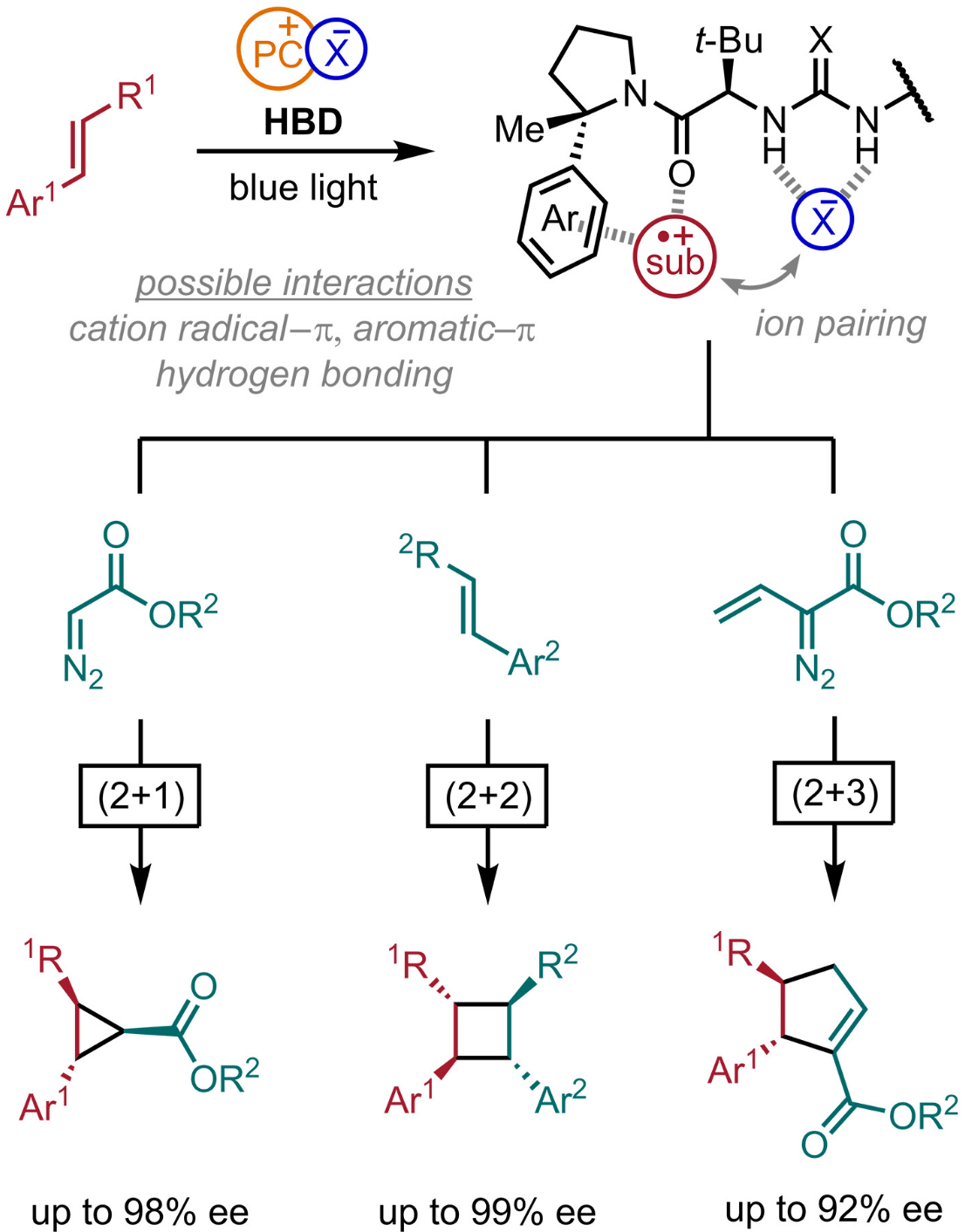
Pairing chiral counteranions with cation radical intermediates provides a potentially generalizable tool for controlling absolute stereochemistry in various reactivity contexts. However, ion-pairing effects on the efficiency of photoinduced processes and the reactivity of radical ion pairs impose severe limits on the chiral anions that can be engaged effectively. In this study, the authors report that association of neutral chiral small-molecule hydrogen-bond donors with the counteranions of cation radical intermediates can achieve enantioselectivity through ion-pairing and other noncovalent interactions. Applications to four different classes of cycloaddition reactions of electron-rich alkene substrates provide cyclic products with up to four new stereocenters in up to 99% enantiomeric excess.

The Asymmetric Synthesis of an Acyclic N-Stereogenic Amine
C. Zhu,† S. Das,† M. S. Sterling, N. Tsuji, S. J. Léger, F. Neese, C. K. De* & B. List*
Nature 2025 (DOI: 10.1038/s41586-025-09905-z)
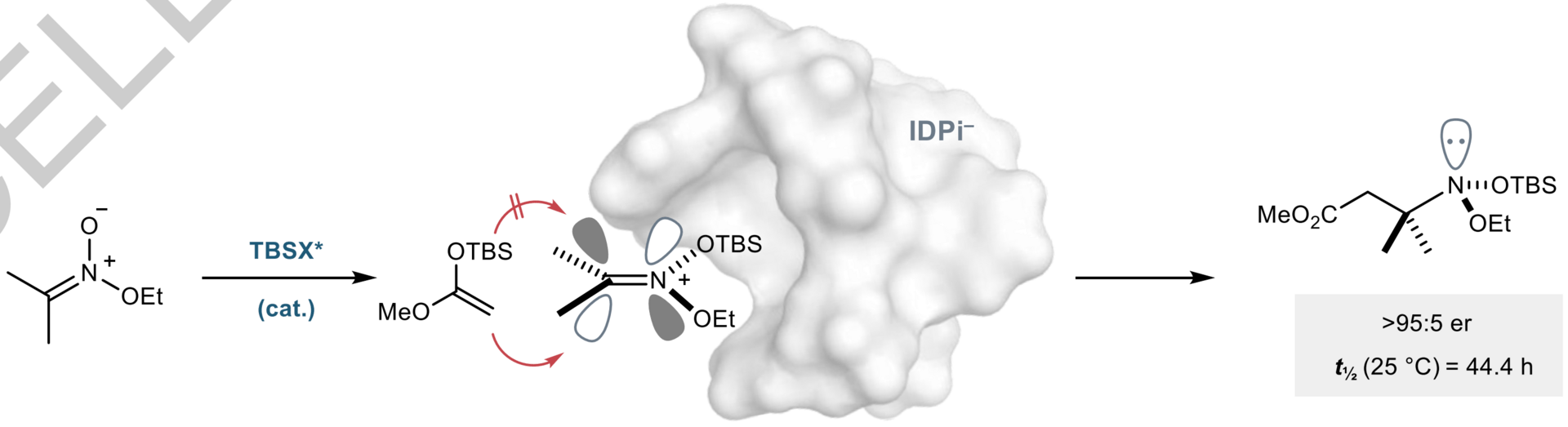
While the synthesis of molecules in which stereogenicity arises from carbon is well established, enantioselective approaches to nitrogen-stereogenic molecules are far less common. In particular, acyclic N-stereogenic amines remain elusive due to rapid pyramidal inversion. Here, the authors report the catalytic asymmetric synthesis of stable, acyclic N-stereogenic amines by the addition of enol silanes to nitronium ions that ion pair to a confined chiral anion. In the produced anomeric amines, the commonly observed isomerization is slowed down by two N-oxy-substituents, which hamper nitrogen inversion.
👉️ For recent, complementary work on “Controlling Pyramidal Nitrogen Chirality by Asymmetric Organocatalysis” by Tan and co-workers, see: here.

Dearomative syn-1,4-Hydroalkylation and C(sp2 )–H Alkylation of Arenes Controlled by Chemoselective Electrolysis
C. Wan, C. Yang, M. Rueping, C. Zhu,* L. Guo* & W. Xia*
Nat. Chem. 2025 (DOI: 10.1038/s41557-025-02001-9)
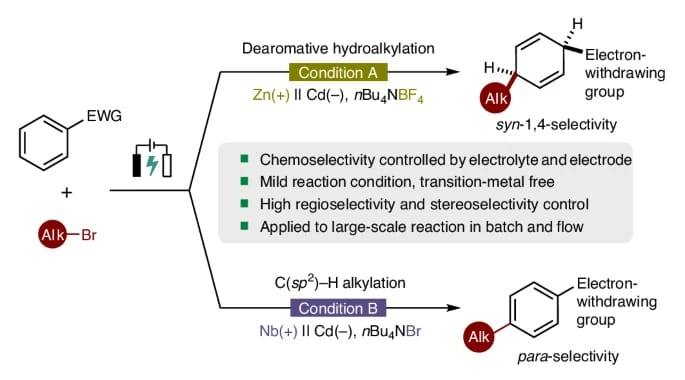
The authors report a dearomative syn-1,4-hydroalkylation reaction of electron-deficient arenes and heteroarenes. This electrochemical approach, conducted under mild, operationally straightforward conditions, facilitates the synthesis of alkylated syn-1,4-cyclohexadienes with high chemoselectivity, regioselectivity and stereoselectivity. In addition, this alkylation protocol is controllable and switchable. By employing a niobium plate as the anode and n-Bu4NBr as the supporting electrolyte, the method enables the para-selective C(sp2 )–H alkylation of (hetero)arenes via electrolysis. Both reactions exhibit broad substrate scope and demonstrate excellent compatibility with various electron-deficient arenes and alkyl bromides.

Late-Stage Conversion of Carboxylic Acids to Nitriles with Mg and Pd Cocatalysis
J. Meng,† M. C. Madhusudhanan,† T. Wang, Z. Cheng, B. Zhao, H. Shi, L. Yang, P. Liu* & N. Jiao*
Nat. Catal. 2025 (DOI: 10.1038/s41929-025-01446-y)
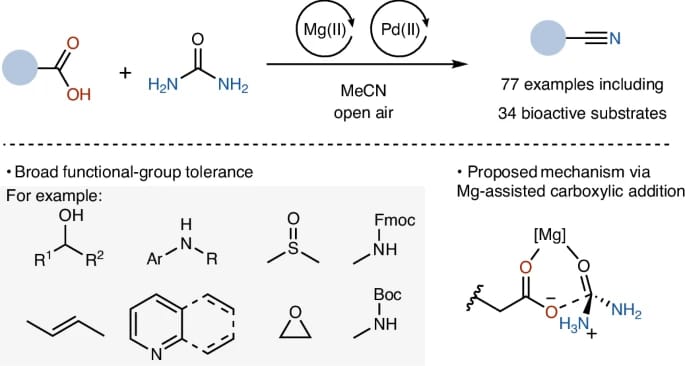
The authors report a mild Mg- and Pd-cocatalysed nitrile synthesis from carboxylic acids with simple, inexpensive urea as the nitrogen source. A pathway involving nucleophilic addition of the carboxylic acid to urea is supported by both mechanistic studies and DFT calculations. The method is effective for the late-stage modification of complex drugs and natural products.
👉️ For recent, complementary work on the “Nitrilation of Carboxylic Acids by PIII /PV -Catalysis” by Radosevich and co-workers, see: here.

Bench-Stable Reagents for Modular Access to Persulfuranyl Scaffolds
R. Li, C. Liu, C. Hu, J. Tsien, M. Hayes, S.-J. Chen, Y. Kanda, R. R. Merchant, B. S. Matsuura & T. Qin*
Nat. Commun. 2025, 16, 10185 (DOI: 10.1038/s41467-025-63621-w) 🔓
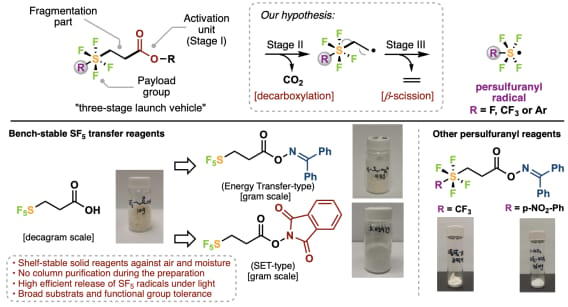
The authors have developed a general pentafluorosulfanylation platform that employs bench-stable solid reagents to generate SF5 radicals via a decarboxylation and β-scission sequence. This strategy enables a variety of operationally simple transformations, expanding the accessibility of SF5-containing molecules. Notably, this reagent design is also adaptable to other persulfuranyl groups, such as trifluoromethyl tetrafluorosulfanyl and aryl tetrafluorosulfanyl groups.

Short, Enantioselective Total Synthesis of (+)-Ineleganolide
K. Yu,* A. Gorou,‡ D. Huang‡ & T. J. Maimone*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c17640) 🔓
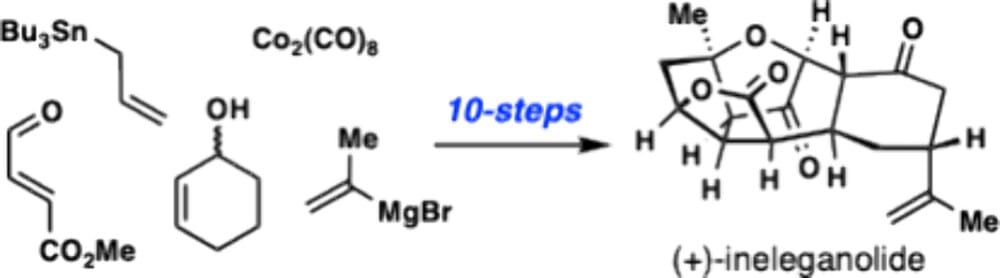
The authors present a 10-step total synthesis of (+)-ineleganolide, a highly oxidized norcembranoid natural product featuring a synthetically challenging caged pentacyclic framework. A trans-selective photochemical [2+2] cycloaddition involving an α,β-unsaturated tricarbonyl chromophore was used to generate a strained cyclobutanol which underwent a C–C bond cleavage cascade when exposed to Brønsted acid. Through this sequence, the ineleganolide core polycycle was generated in only six steps from commercially available materials.
Dual Catalytic Enantioconvergent Carbamoylation of Aziridines
L. Zhang,† H. Liu,† T. G. Santiago & R. Martin*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c15873) 🔓
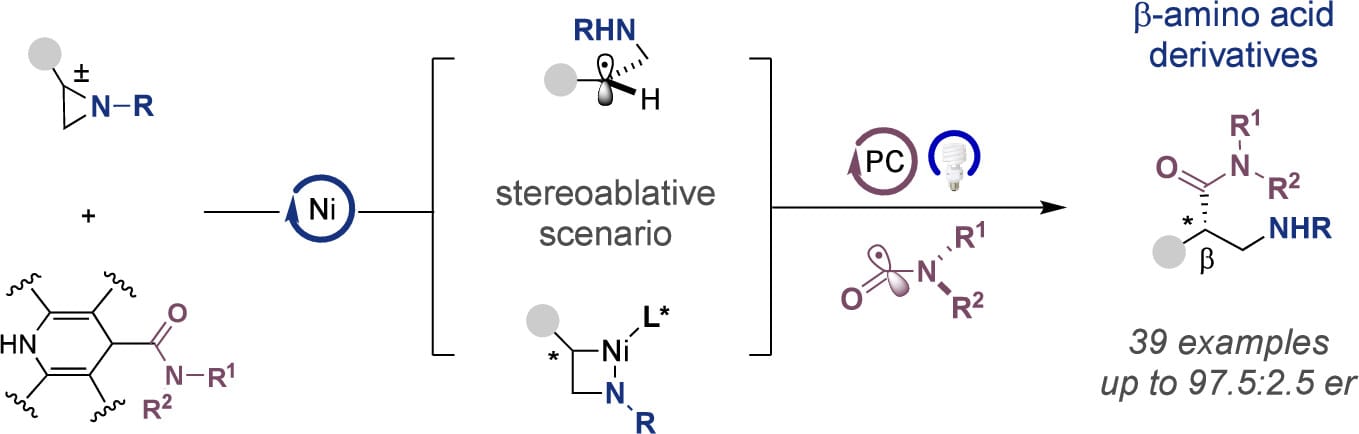
The authors disclose an enantioconvergent carbamoylation of racemic aziridines enabled by a dual catalytic strategy. This method offers a new entry point to highly enantioenriched β-amino amides from simple precursors and is characterized by its broad applicability, even with challenging combinations. Preliminary studies with well-defined nickel complexes support a scenario via carbamoyl organometallic intermediates.
Cobalt-Catalyzed Photoelectrochemical Dehydration of Primary Alcohols
X. Ying, Y. Zhang & C. Li*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c15270)
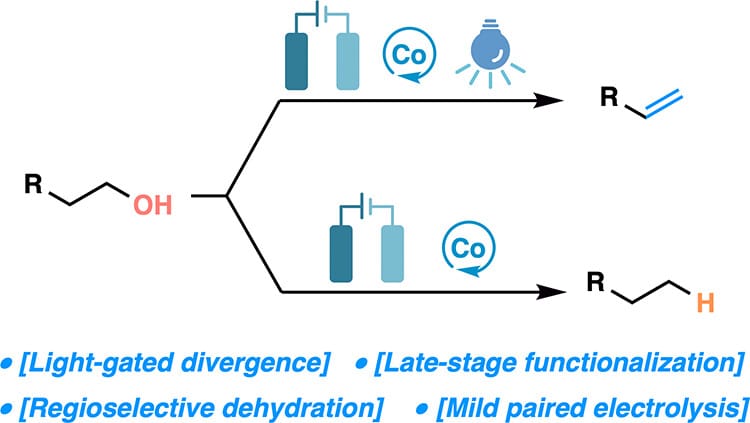
The authors report a cobalt-catalyzed, photoelectrochemical strategy for the direct conversion of primary alcohols to terminal alkenes. The reaction proceeds in an undivided cell under blue-light irradiation, employing a readily available CoCl2/dimethylglyoxime (dmgH2) catalyst system. In contrast, without irradiation, the same conditions yield deoxygenated alkanes. Both methods display broad substrate scope, high regioselectivity across polyol frameworks, and exceptional tolerance of oxidation-sensitive functional groups.
Markovnikov-Selective Hydroboration of Aryl Alkenes Catalyzed by Quaternary Ammonium Salts
P. Huninik, H. Im, J. Szyling, M.-H. Baik* & J. Walkowiak*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c13555)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2024-zf116) 🔓
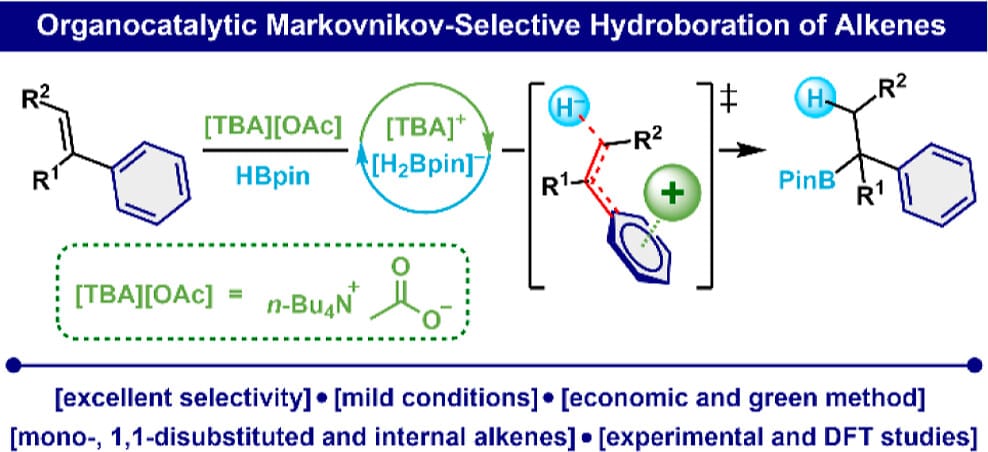
The authors present an organocatalytic method for the Markovnikov-selective hydroboration of aryl alkenes using a readily available quaternary ammonium catalyst. This approach is operationally simple, scalable, and demonstrates broad substrate compatibility. Furthermore, the method enables the efficient synthesis of active pharmaceutical ingredients, including Chlorphenoxamine.
Bulky Phosphine Ligands Promote Palladium-Catalyzed Protodeboronation
C. T. Ser,† H. Hao,† S. Pablo-García, K. Jorner, S. Li, R. Pollice* & A. Aspuru-Guzik*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c14153)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2024-cw8cs-v3) 🔓
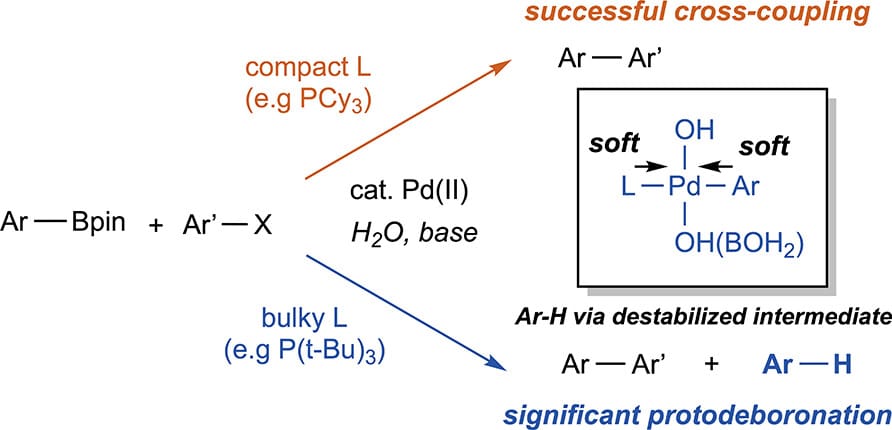
The Suzuki–Miyaura cross-coupling reaction is plagued by protodeboronation, an undesirable side reaction with water that consumes boronic acid derivatives. Mechanistic studies have established protodeboronation to be sensitive to the nature of the boronic reagent, the reaction conditions, and in particular, the presence of bases. However, protodeboronation catalyzed by palladium-phosphine complexes is less studied. Here, the authors report that protodeboronation is accelerated by palladium(II) complexes bound to bulky phosphine ligands. While sterically hindered ligands are used to facilitate difficult cross-couplings, these ligands can instead paradoxically impede cross-coupling product formation.

Direct Access to Acylboranes via Formal Condensation of Carboxylic Acids with Ligated Boranes
C.-Y. Cai, C. Hu, K. E. Colbaugh, R. R. Merchant, S. Chen* & T. Qin*
Angew. Chem. Int. Ed. 2025, Early View (DOI: 10.1002/anie.202516826) 🔓
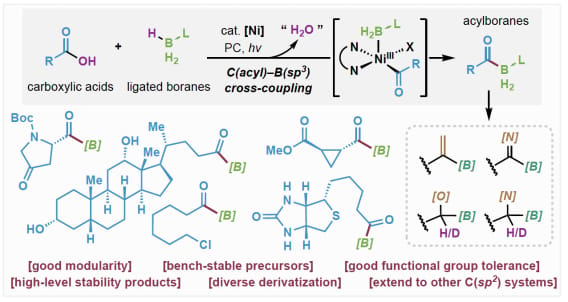
A nickel-catalyzed, radical-based C(acyl)–B cross-coupling strategy has been developed for the efficient synthesis of bench-stable, phosphonite-ligated acylborane compounds from readily available carboxylic acids. This methodology not only provides access to acylboranes with diverse reactivity profiles but also establishes a versatile platform for the direct construction of stabilized organoborane species, which has currently been extended to C(aryl) systems.

Development of Multiple Local Computational Models in Retrosynthetic Analysis: Total Synthesis of (−)-Deoxylimonin
L. M. Gharbaoui,† J. Eun,† Y. Zhao & T. R. Newhouse*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-lj989) 🔓
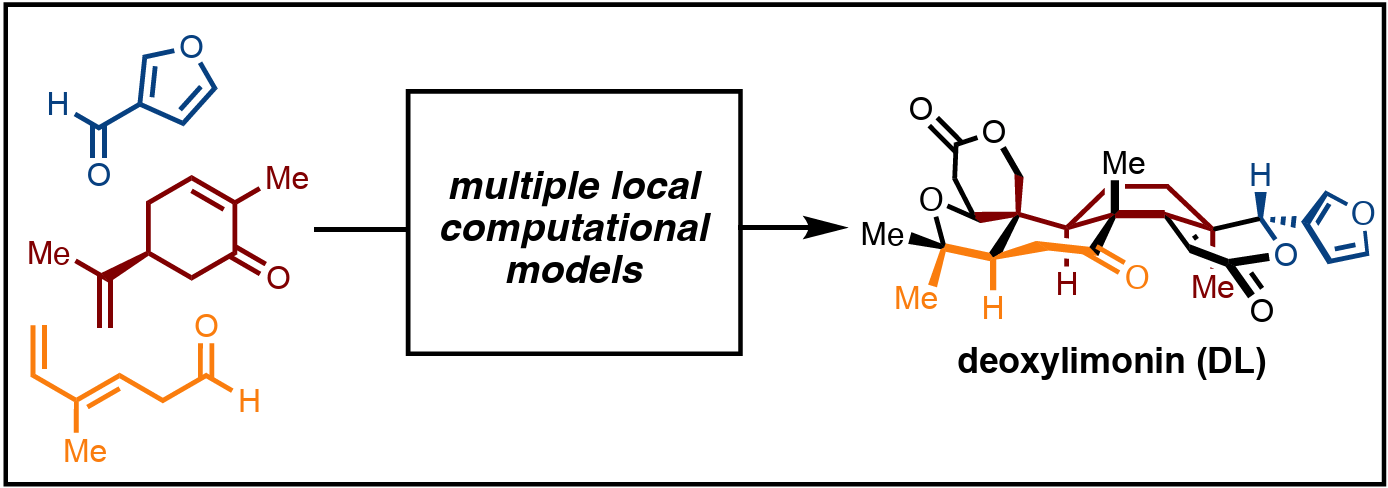
This work describes the development of a de novo strategy to access the A,D-seco limonoids utilizing robust synthetic operations allowing for the synthesis of a characteristic member, deoxylimonin. The retrosynthetic strategy uses resilient chemistry for the assemblage of a key carbocyclic tricycle in only 8 synthetic maneuvers. This intermediate is then amalgamated through a modern variation on the classic combination of ring cleavage and formation using radical attack at oxygen in a key synthetic operation. Two unique local computational models were developed and rapidly integrated into the design stages of retrosynthetic analysis illustrating how multiple local models augment the intersection of transform-based and structure-goal based approaches.
Concise Synthesis of Deoxylimonin
J. Bao,† L. Yao,† H. Tian & J. Gui*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-23nhc) 🔓
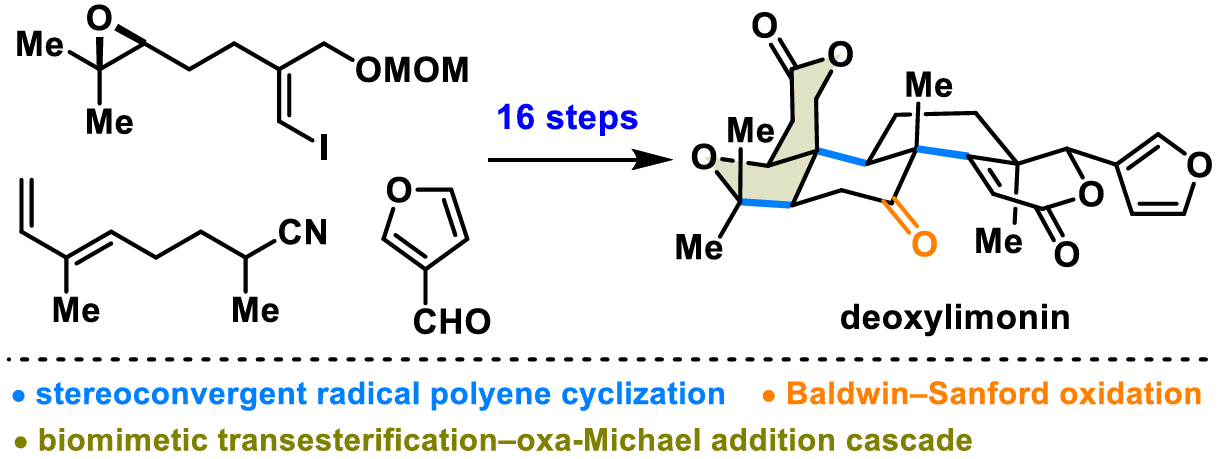
The authors present a concise synthesis of the flagship member of limonoids, deoxylimonin, by a bioinspired skeletal reorganization approach. Key features of this synthesis include a stereoconvergent radical polyene cyclization to assemble the 6/6/6 tricyclic system, an oxime-directed Baldwin–Sanford oxidation to hydroxylate the unactivated C7–H, a titanium-mediated intermolecular aldol reaction to introduce the 3-furanyl lactone moiety and two contiguous stereogenic centers, and a biomimetic transesterification–oxa-Michael addition cascade to install the tetrahydrofuran-δ-lactone-fused motif.
Radical Retrosynthesis of Natural Products Enabled by Iron-based Reductive Olefin Coupling
G. L. Barnes,† A. L. Rerick† & P. S. Baran*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-84gsv) 🔓
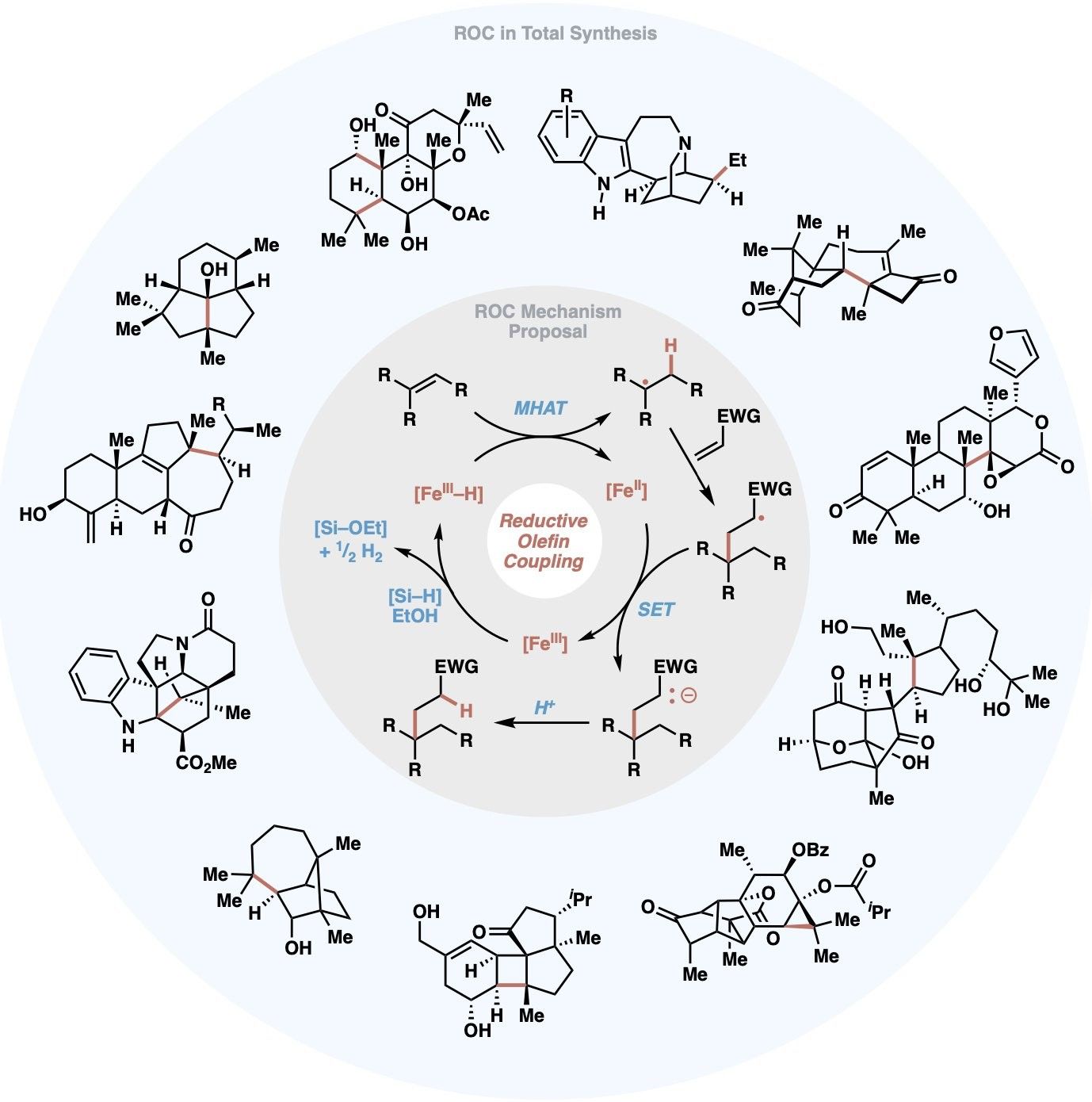
This review highlights iron-catalyzed reductive olefin coupling (ROC)—a versatile metal-hydride hydrogen atom transfer (MHAT)-driven strategy for C–C bond formation—as a key tool in the radical retrosynthesis for natural products. Initiated by the 2014 disclosure of Fe-catalyzed inter- and intramolecular olefin–olefin coupling, ROC combines Mukaiyama's hydrofunctionalization with Giese's radical additions. Emphasizing strategic disconnections, the review surveys dozens of total syntheses across terpenoids, alkaloids, and polyketides. The many applications of ROC highlight its power to forge strained rings, quaternary centers, and stereocenters from olefins, often supplanting 2e− tactics. Troubleshooting guides and retrosynthetic blueprints are included to aid future applications in complex molecule assembly.

Reduction and Deuteration of N-Heteroarenes Using an Organic Photoredox Catalyst
A. K. Bains, A. Sau, B. S. Portela, R. S. Paton,* G. M. Miyake* & N. H. Damrauer*
Chem 2025, Online Now (DOI: 10.1016/j.chempr.2025.102822)
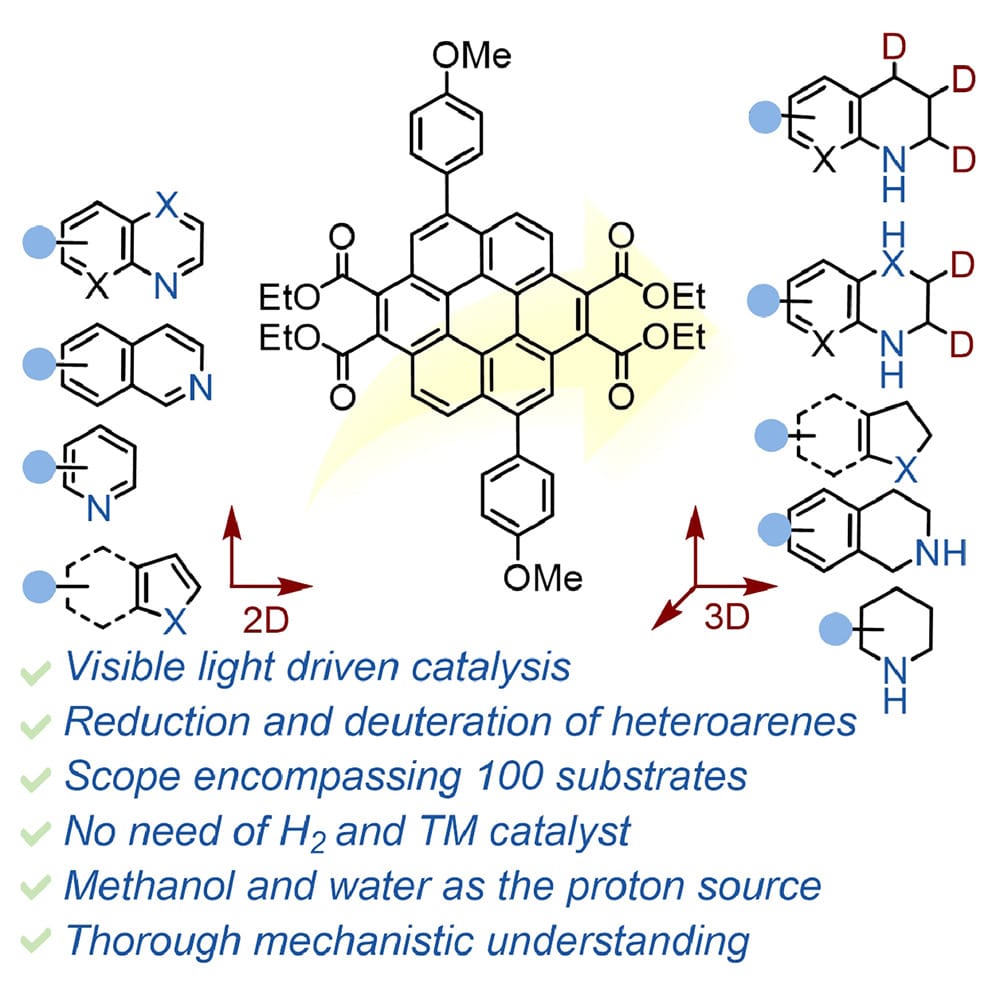
The authors introduce core-extended coronene-tetraesters as organic photoredox catalysts for the photoinduced reduction and deuteration of a range of heteroarenes at ambient temperature using visible light from commercially available LEDs. This protocol accommodates over nine types of heteroarenes under a common reaction condition.

Selective Conversion of Esters to Ketones via β-Ketosulfone Intermediates
E. Fung & P. S. Fier*
Org. Lett. 2025, ASAP (DOI: 10.1021/acs.orglett.5c04381)
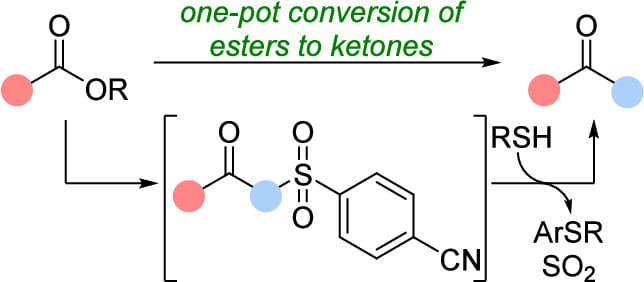
The authors report a general and practical strategy for the conversion of esters to ketones using electron-deficient aryl sulfones. This method is operationally simple, proceeds under mild conditions, and enables the conversion of both aryl and enolizable alkyl esters to alkyl ketones in good to excellent yields. Electrophilic trapping of the β-ketosulfone intermediates enables access to a variety of ketones from a single precursor.

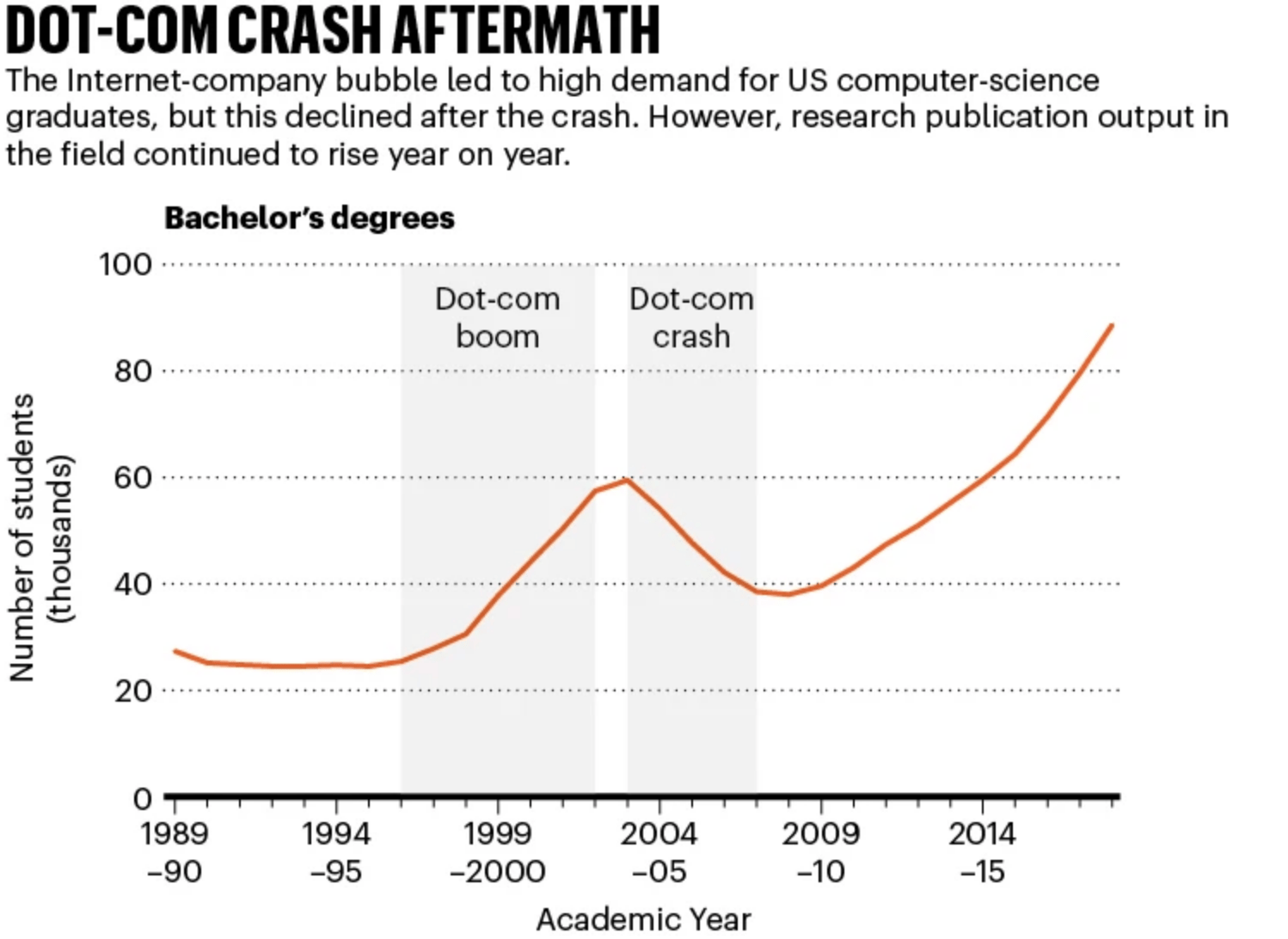
Boom and Bust
🤖 Boom and Bust. The scale of today’s AI boom is extraordinary, easily eclipsing the dot-com boom, when the Nasdaq-100 $QQQ ( ▲ 0.89% ) climbed more than 600% before crashing 78% between 2000 and 2002, wiping out $5 trillion in stock market value. In an interesting parallel, AI-chipmaker Nvidia $NVDA ( ▲ 1.02% ) recently surged beyond $5 trillion in valuation, briefly making it worth more than the GDP of every country except the US and China.
This rapid acceleration in AI-related company valuations has analysts worried that an “AI bubble” may be forming, similar to the dot-com boom or even the “bike boom” of 1896, when share prices in British bicycle companies tripled within months and the number of manufacturers soared before a wave of cheaper American imports undercut the market, causing around 70% of British bicycle firms to collapse by 1901. Yet the technological advances from this period directly seeded motorcycles, cars, and even early aviation, much like the dot-com bubble helped pave the way for high-speed internet infrastructure, cloud computing, e-commerce platforms, and a generation of digital pioneers.
Economists often highlight periods like this to show that speculative bubbles can destroy capital while still accelerating transformative technologies. However, investment in AI is now dramatically higher than investment in internet companies during the build-up to the dot-com crash—17× higher, in fact. Yet, a McKinsey report found that nearly 80% of companies using AI have seen no meaningful boost to earnings, and researchers continue to highlight the limitations of current models.
If a crash occurs, experts suggest the fallout could even exceed the impact of the dot-com collapse. However, evidence suggests that research activity will remain resilient. During the dot-com bubble, computer-science publications continued to rise, and displaced talent helped fuel innovation in other sectors. A similar pattern may emerge in AI: researchers leaving industry could strengthen academic labs or launch new scientific ventures.
NB: According to recent reports, around 80% of Nvidia employees are now millionaires, thanks in large part to the company’s employee stock purchase programme, with roughly 50% of employees reportedly having a net worth exceeding $25 million.
That’s all for this issue! Have a great week and we’ll see you next Monday.
Reply