- Synthesis Spotlight
- Posts
- Light, Enzymes, Action
Light, Enzymes, Action
💡 How Allergens Breach Airway Defences

Monday 28th July – Sunday 3rd August 2025 | Volume 2, Issue 30 |


Diversity-Oriented Photobiocatalytic Synthesis via Stereoselective Three-Component Radical Coupling
C. Zhang,† J. Zhou,† P.-P. Xie, S. M. Rivera, T. M. Alturaifi, J. Finnigan, S. Charnock, P. Liu* & Y. Yang*
Science 2025, First Release (DOI: 10.1126/science.adx2935)
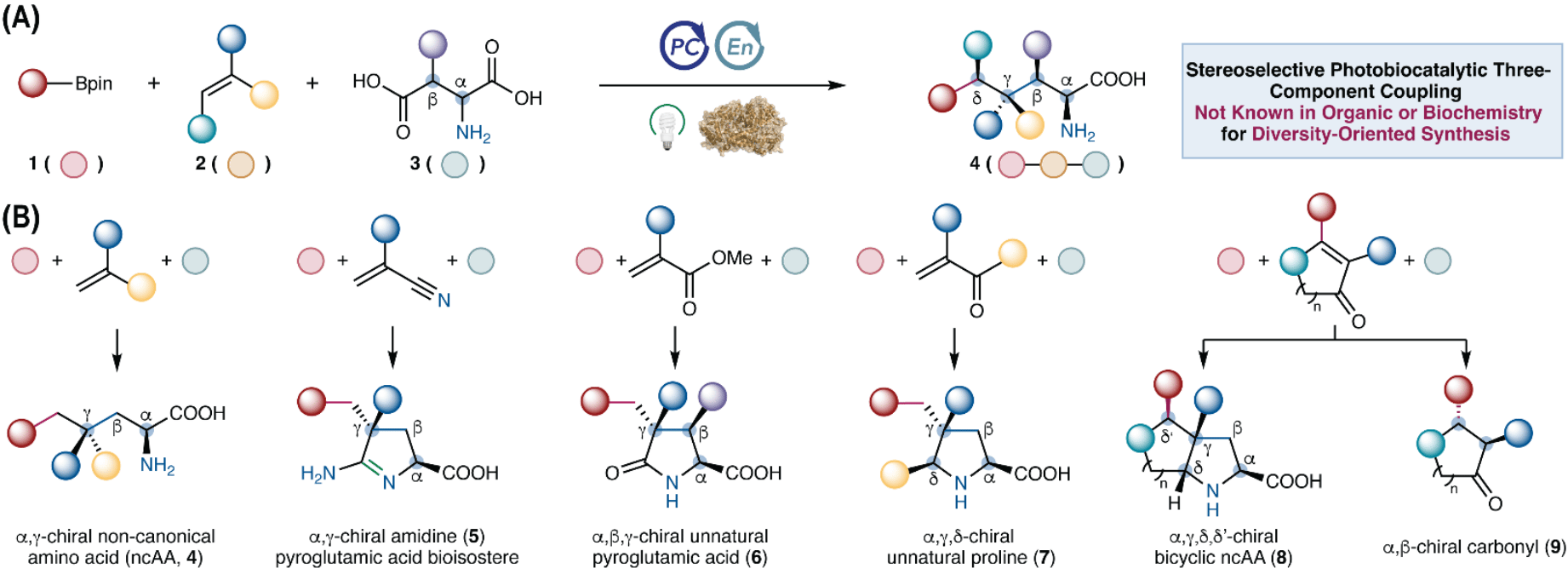
Using cooperative photobiocatalysis, the authors developed a stereoselective three-component radical-mediated C–C coupling unknown in both organic chemistry and biochemistry. Directed evolution of repurposed pyridoxal decarboxylases enabled full fragment variability in this three-component coupling, giving rise to six classes of valuable products, many of which were inaccessible by other methods, even in a racemic fashion. The broad substrate scope and complementary specificities of evolved enzyme variants enabled combinatorial library synthesis, affording structurally and stereochemically diverse scaffolds for medicinal chemistry.

Skeletal Editing of 4-Arylpyrimidines into Diverse Nitrogen Heteroaromatics via Four-Atom Synthons
S. Li,† Y. Shi,† J. Tang, M. Yan, S. Huang, T. Dou, S. Wang, X. Jin, Z. Su, W. Jiang, J. Xu, X. Zheng, R. Li, H. Chen, W. Xue* & H. Fu*
Nat. Commun. 2025, 16, 7112 (DOI: 10.1038/s41467-025-62547-7) 🔓

The authors present a scaffold hopping strategy for the skeletal editing of pyrimidines into a range of heteroarenes through the addition of nucleophiles, ring-opening, fragmentation, and ring-closing (ANROFRC) processes. The method features the in situ generation of a vinamidinium salt intermediate, which serves as a unique N-C-C-C four-atom (A4) synthon that reacts with A1 and A2 synthons.

Harnessing O-Vinylhydroxylamines for Ring-Annulation: A Scalable Approach to Azaindolines and Azaindoles
Z. Grimm,† C. Randolph,† O. Buravov, P. Mykhailiuk* & L. Kürti*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c06568)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-4p67k) 🔓
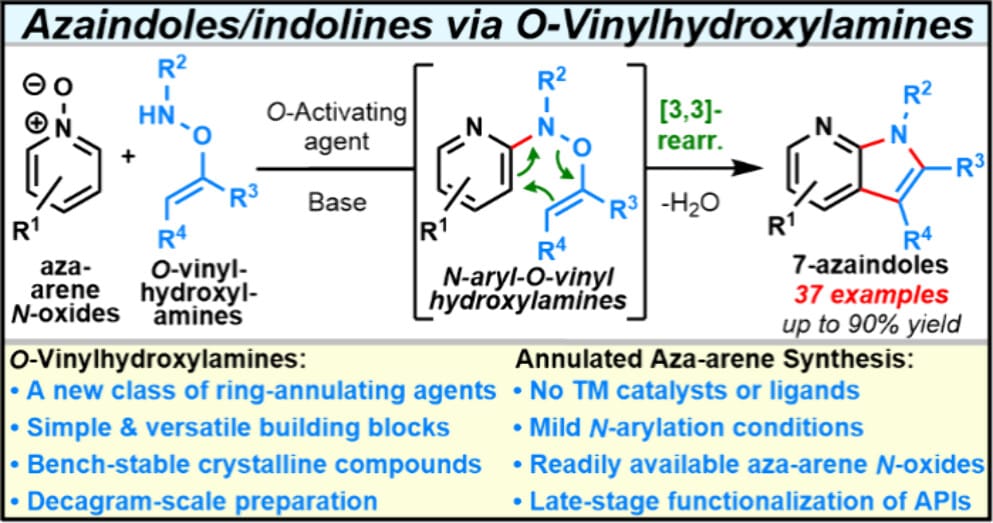
The authors report a convenient and scalable strategy for the synthesis of 7-azaindolines and 7-azaindoles using O-vinylhydroxylamines as ring-annulation reagents. In this approach, O-vinylhydroxylamines undergo N-arylation with aza-arene N-oxides, triggering a rapid [3,3]-sigmatropic rearrangement, which is followed by rearomatization and cyclization to deliver 7-azaindolines that can be readily dehydrated to afford the corresponding 7-azaindoles. The method offers a broad substrate scope, enabling access to an array of highly functionalized derivatives, and the mild reaction conditions facilitate late-stage functionalization of complex molecules.
Asymmetric C–H Functionalization of N-Boc-2,5-dihydro-1H-pyrrole and Its Application as a Key Step in the Synthesis of (−)-Dragocin D
T.-T. H. Nguyen, K. Shimabukuro, D. G. Musaev, J. Bacsa, A. Navarro & H. M. L. Davies*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c08080) 🔓

Dirhodium tetracarboxylate-catalyzed reaction of aryldiazoacetates with N-Boc-2,5-dihydro-1H-pyrrole results in a highly enantio- and diastereoselective C–H functionalization exclusively at the α-N C2 position—in sharp contrast to the reaction with ethyl diazoacetate, which results in cyclopropanation of the olefinic site. Rh2(S- or R-PTAD)4 is the optimal chiral catalyst and is capable of generating the C–H functionalization products in up to 87% yield with high levels of diastereoselectivity (>20:1 d.r.) and enantioselectivity (97% e.e.) with a low catalyst loading (0.05 mol%). The utility of the C–H functionalization chemistry was illustrated by its application to the synthesis of (−)-dragocin D and a variety of pharmaceutically relevant pyrrolidines.
anti-Diastereo- and Enantioselective Ruthenium-Catalyzed C–C Coupling-Oxidative Lactonization of 1,4-Butanediol: Alkynes as Allylmetal Pronucleophiles
C. G. Santana, P. W. Meares,‡ Z. J. Dubey‡ & M. J. Krische*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c09358) 🔓

Using the ruthenium catalyst assembled from RuHCl(CO)(PPh3)3, JOSIPHOS SL-J009-1 and KI, allylative oxidative lactonizations of 1,4-butanediol are described that occur with high levels of anti-diastereo- and enantioselectivity. These processes merge three distinct catalytic events: (i) alkyne-to-allene isomerization, (ii) transfer hydrogenative carbonyl (α-aryl)allylation of the allene pronucleophile, and (iii) oxidative lactonization of the resulting enantiomerically enriched 1°,2°-diol.
Modular Synthetic Platform for the Elaboration of Fragments in Three Dimensions for Fragment-Based Drug Discovery
A. R. Gomez-Angel,† H. F. Klein,† S. Y. Yao,† J. R. Donald, J. D. Firth, R. Appiani, C. J. Palmer, J. Lincoln, S. C. C. Lucas, L. Fusani, R. I. Storer & P. O’Brien*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c08786) 🔓

The authors present a modular platform for the elaboration of 2D fragment hits into lead-like 3D compounds, utilizing nine bifunctional building blocks that each access a distinct 3D exit vector. The building blocks comprise: (i) rigid sp3 -rich bicyclic cyclopropane-based structures to fix the vectors and (ii) two synthetic handles—a protected cyclic amine and a cyclopropyl N-methyliminodiacetic acid (MIDA) boronate. Starting from the drug Ritlecitinib, the development of inhibitors of Janus kinase 3 (JAK3) around a putative pyrrolopyrimidine 2D fragment hit was explored, streamlining the discovery of a novel and selective JAK3 inhibitor with IC50 = 69 nM.
Leveraging Data Science to Elucidate Ligand Features for Pd-Catalyzed Enantioretentive N-Arylations of Cyclic α-Substituted Amines in Aqueous Media
A. R. Ickes,† J. P. Liles,† N. Borlinghaus, J. Henle, R. Swiatowiec, N. P. Kaushik, W. M. Braje, K. C. Harper, S. Shekhar* & M. S. Sigman*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c07224)

The authors disclose a method for the enantioretentive N-arylation of cyclic secondary amines. After an extensive HTE campaign, a novel phosphorinane ligand for palladium, “NiniPhos”, was found to demonstrate high yields (up to 96%) and enantiospecificity (96 to >99% e.s.) across a range of amines with diverse aryl halides under aqueous conditions. Despite screening more than 120 ligands, only 31 demonstrated catalytic activity (>10% yield), resulting in a highly skewed data set. By applying data science-driven hotspot analysis and machine learning (linear support vector machines), the structural features of ligands leading to the activity cliff were identified, pinpointing di-tert-butylphosphines and phosphorinanes as privileged ligand classes in this reaction.
Regulating the Acidity of HF/Pyridine Media to Control the Chemodivergent Fluoro- and Hydro-Heteroalkylation of Olefins
M. Longuet,† F. Naullet,† A. Pérochon,† A. Martin-Mingot, F. Guégan,* B. Michelet* & S. Thibaudeau*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c07526)

Oxocarbenium ions are important reactive intermediates with many applications; however, primary oxocarbenium ions have been much less explored, in part due to the difficulty of controlling their reactivity under standard conditions. Here, the authors show that the reactivity of such species can be finely modulated in strongly acidic HF/pyridine media. By leveraging the tunable acidity of HF/pyridine solutions, their reaction with olefins can be diverted toward either fluoro- or hydro-oxyalkylation.
Directed Halogen Atom Transfer (DIXAT): A Powerful Tool for Chemoselective Generation of Aryl Radicals Toward Remote C(sp3 )–H Functionalization of Aliphatic Amines
S. Wagulde, K. P. Quirion, T. M. Alturaifi, P. Liu* & V. Gevorgyan*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c09218)

The authors report a directed halogen atom transfer (DIXAT) that enables the chemoselective activation of aryl bromides bearing an ortho directing group, even in the presence of otherwise more reactive aryl iodides. This novel technique was harnessed to conduct nickel-catalyzed arylation and cascade carboarylation of the remote C(sp3 )–H bonds of aliphatic amines. The transformation features operational simplicity, high functional group tolerance, a broad scope, and applicability to late-stage C(sp3 )–H functionalization.
Enantioselective Synthesis of Spirocyclic Nitrogen-Containing Heterocycles Catalyzed by an Iridium-Containing Cytochrome
J. Xu,† B. J. Bloomer,† J. N. Brunn, A. P. Quest, S. Chakraborty, J. E. Schneider, D. S. Clark & J. F. Hartwig*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c06239)

The authors report the stereoselective cyclopropanation of methylene-substituted saturated heterocycles catalyzed by an iridium-containing cytochrome. After just four rounds of mutagenesis, a variant was identified that forms spiroazetidines, spiropyrrolidines, and spiropiperidines with enantioselectivities up to 99%.
N-Heterocyclic Selone-Catalyzed [2σ+2π] Cycloaddition of Bicyclo[1.1.0]butanes via Photoredox Radical Buffer
K. Lin, C.-R. Luo-Wei, Y. Gao, J. Lan & T. Zhu*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c05306)

The authors disclose novel redox-active N-heterocyclic selone (NHS) catalysts that enable a radical-catalyzed [2σ+2π] cycloaddition platform, providing direct access to 1,3-bicyclohexane, a bioisostere for meta-substituted benzene. The method enables efficient radical-mediated cycloaddition of vinyl-bicyclobutanes (V-BCBs) with diverse alkenes, achieving high yields (up to 99%) and broad substrate compatibility (47 examples) with a low catalyst loading (1 mol%).

Thianthrenium Salt-Mediated Homologative 1,3-Dielectrophilic Activation Strategy for Alkene Difunctionalization
S. Paul,† A. Richardson,† L. Buck, G. Centonze, R. Coccia, F. Sardelli & M. Silvi*
Angew. Chem. Novit 2025, 1, e70005 (DOI: 10.1002/anov.70005) 🔓

The authors disclose a thianthrenium salt-mediated homologative dielectrophilic activation method for alkenes with wide functional group tolerance. The strategy enables access to 1,3-diazides, diamines, diols, dihalides, dithiols as well as to the full matrix of their heterodifunctionalized products. At the core of this work is the design of a novel sulfonium atom-transfer reagent, which undergoes selective bond cleavage to reveal a methyl thianthrenium radical.
Catalytic Enantioselective Diboration of 1,2-Dihydropyridines
H. Liang & J. P. Morken*
Angew. Chem. Int. Ed. 2025, Early View (DOI: 10.1002/anie.202511813)
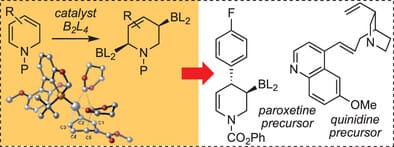
Dihydropyridines undergo enantioselective 1,4-diboration in the presence of a chiral Pt-based catalyst. The resulting diboryl piperidines can undergo diverse stereospecific cross-couplings, allowing the construction of an array of different substituted chiral piperidines. DFT calculations provide insight into a plausible diboration pathway that involves a platinum-centered reaction between coordinated B2L2 reagent and the diene to furnish an intermediate π–allyl platinum complex.
Privileged Chiral Photocatalysts
E. Studer,† S. Mandal,† T. Stünkel & R. Gilmour*
Angew. Chem. Int. Ed. 2025, Early View (DOI: 10.1002/anie.202513320) 🔓

Privileged chiral catalysts have transformed asymmetric synthesis, conferring generality to processes that are routinely leveraged in the construction of societally important functional small molecules. This mini-review is intended to survey the conception and evolution of privileged chiral photocatalyst scaffolds that enable simultaneous orchestration of reactivity and enantioselectivity in non-ground state regimes.

A General Strategy for Light-Mediated Nickel-Catalyzed C(sp2 )-Heteroatom Cross-Couplings
A. R. Bena, T. Banik,‡ C. Giannoudis,‡ F. Ortis,‡ D. Bím, G. H. Palissery & B. Pieber*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-1czpp) 🔓

Light-mediated NiI /NiIII catalysis presents a promising alternative to thermal Pd- and Ni-catalyzed C(sp2 )–heteroatom cross-couplings that operate through M0 /MII mechanisms and rely on complex ligands. However, existing methods often require high nickel loadings, exhibit a limited scope, employ substrate-specific conditions, and use exogenous photocatalysts. Here, the authors report a broadly applicable catalytic system that addresses these challenges through the design of a readily accessible ligand, which facilitates oxidative addition on NiI . A photocatalyst is not necessary because the applied base serves the dual role of enabling precatalyst activation with visible light. This approach simplifies late-stage functionalization of complex molecules and enables gram-scale synthesis of pharmaceutical targets with catalyst loadings as low as 100 ppm.
Three-Component Assembly and Structure-Function Relationships of (–)-Gukulenin A
V. Gupta, Z. Wang, J. B. Combs, T. Wright, L. Chen, B. Lin, R. Holmes, B. Qin, J. Oh, J. M. Crawford & S. B. Herzon*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2023-8dj1k-v3) 🔓

(–)-Gukulenin A is a cytotoxic secondary metabolite that bears two aromatic α-tropolone residues, 10 asymmetric stereocenters, functionalized cyclopentane fragments, a labile hemiketal, and an electrophilic aldehyde. Here, the authors describe its enantioselective synthesis by a three-component assembly strategy. Key steps include the directed C–H arylation of a norbornyl picolinamide, a tandem Grob fragmentation–alkylation, a novel method for the synthesis of α-tropolones, a site-selective, multicomponent cross-coupling reaction, and a thermal carbonyl–ene reaction to complete the carbon skeleton. (–)-Gukulenin A is efficacious and well-tolerated in murine models of ovarian cancer, and structure-function studies establish the dimeric tropolone and aldehyde substructures as critical drivers of cytotoxicity.
Cu/Ir-Cooperative Catalysis for the Enantioselective Borylallylation of Electron Deficient Alkenes
S. Das, S. K. Dorn & M. K. Brown*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-4xjpv) 🔓

The authors present a Cu/Ir-catalyzed borylallylation of electron deficient alkenes that operates by the cooperative function of a chiral Cu-complex and a chiral Ir-complex to generate products with high levels of stereocontrol. The method allows for the stereodivergent synthesis of acyclic products with control of three stereogenic centers. Importantly, the products contain C–B, ester, and alkene functionalities, which allows for a diverse range of products to be generated.

Pore-Forming Proteins Trigger Allergic Responses
🍃 Pore-Forming Proteins Trigger Allergic Responses. A new study in Nature has challenged our understanding of how allergens trigger immune responses. Traditionally, research has focused on how individual allergens, such as pollen or pet dander, provoke inflammation. Now, a team in Beijing has uncovered a more general mechanism: certain allergens literally poke holes in the cells that line our airways.
By isolating two pore-forming proteins (Aeg-S and Aeg-L) from the mould Alternaria alternata, the researchers found that these proteins team up to form pores in the membranes of airway epithelial cells. This results in a rush of calcium ions into the cells, setting off an immune response and leading to the usual suspects: sneezing, itchy eyes, and coughing.
The work offers a compelling shift in how we think about allergens and therapies that block these pore-forming proteins could offer broad-spectrum allergy relief.
That’s all for this issue! Have a great week and we’ll see you next Monday.

Reply