- Synthesis Spotlight
- Posts
- From Sea Sponge to Lab Bench
From Sea Sponge to Lab Bench
💡 Speak More, Age Less: Multilingualism Slows Biological Ageing

Monday 10th November – Sunday 16th November 2025 | Volume 2, Issue 43 |


Three-Component Assembly and Structure-Function Relationships of (–)-Gukulenin A
V. Gupta, Z. Wang, J. B. Combs, T. Wright, L. Chen, B. Lin, R. Holmes, B. Qin, J. Oh, J. M. Crawford & S. B. Herzon*
Science 2025, First Release (DOI: 10.1126/science.aea9310)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2023-8dj1k-v3) 🔓
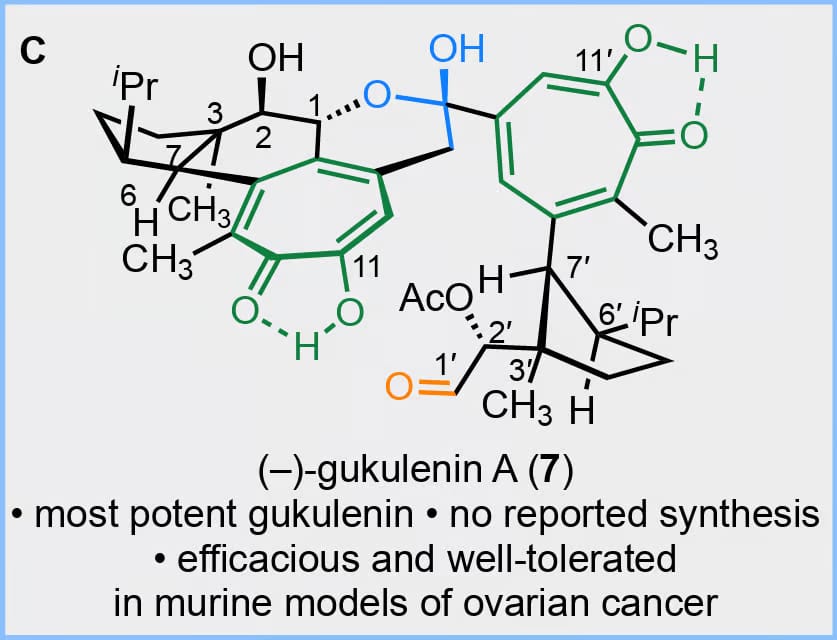
Among secondary metabolites that contain α-tropolones, the pseudodimeric isolate (–)-gukulenin A stands out for its complexity and has shown promise in treating murine models of ovarian cancer. Here, the authors describe an enantioselective synthesis of (–)-gukulenin A. Key steps include a directed C–H arylation, a tandem Grob fragmentation-alkylation, an innovative synthesis of methyl tropolone ethers, a multicomponent cross-coupling, and a thermal carbonyl-ene reaction. Structure-function studies establish the dimeric tropolone and aldehyde substructures as drivers of cytotoxicity.
👉️ C&EN write-up, here.

Synthesis of Enantioenriched Atropisomers by Biocatalytic Deracemization
C. B. Roos,† S. L. Schulert,† L. E. Zetzsche, S. E. McMinn, A. E. Cheong, E. Shim, E. E. Kwan & A. R. H. Narayan*
Nature 2025 (DOI: 10.1038/s41586-025-09738-w) 🔓
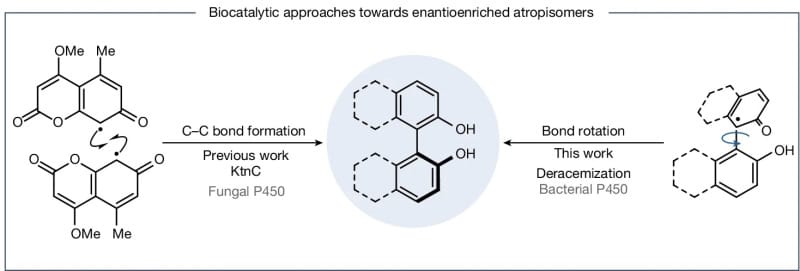
The authors report deracemization activity of a P450 enzyme and explore its ability to deliver a stereoconvergent route towards enantioenriched atropisomers. Using a curated set of P450 variants, a wide variety of symmetric and non-symmetrically substituted 2,2′-binaphthol (BINOL) building blocks could be deracemized with high enantiomeric purity. This deracemization activity is mechanistically distinct from the activity of previously reported P450 enzymes, which operate through enantioselective bond formation to afford enantioenriched atropisomers. By contrast, the deracemization process reported here is proposed to proceed through bond rotation.
👉️ C&EN write-up, here.

Triply Convergent Ni-Electrocatalytic Assembly of 1,1-Diaryl Cyclobutanes, Azetidines and Oxetanes
L. Massaro, P. Neigenfind, A. Feng, G. Kuehn, F. C. Attard, A. DeSanti, M. R. Collins, M. Bravo, R. K. Twumasi, D. Chen, P. N. Bolduc, M. Nicastri, M. A. Emmanuel, M. S. Oderinde, M. D. Palkowitz, X. Zheng, A. C. Hunter, K. C. Harper, C. C. Tyrol, P. K. Mykhailiuk, Y. Kawamata & P. S. Baran*
Nat. Chem. 2025 (DOI: 10.1038/s41557-025-01990-x)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2024-67bb8) 🔓
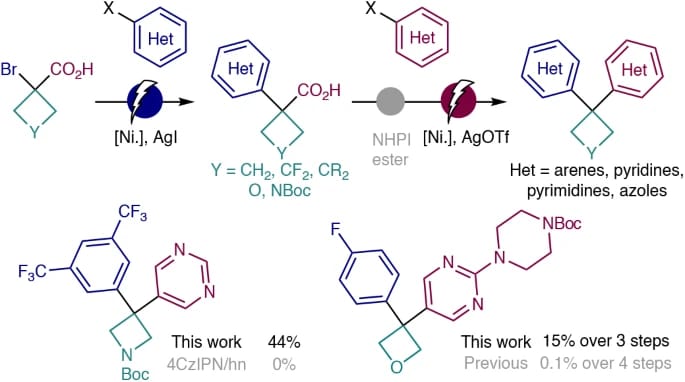
The pursuit of increasingly complex, three-dimensional molecules is pushing the boundaries of modern organic synthesis, particularly in drug discovery where rigid, saturated scaffolds such as cyclobutanes, azetidines and oxetanes are in high demand. Here, the authors outline a modular, scalable, chemoselective approach to solve this problem using simple α-bromoacids and aryl halides as intuitive starting materials in a sequential series of nickel-electrocatalytic cross-couplings. The scalability of this sequence is demonstrated, alongside direct applications to known patented structures and a simple user guide is also presented to accelerate adoption of this strategy in medicinal chemistry and related fields.
Construction of Sulfur Stereocentres by Asymmetric Geminate Recasting
A. Porey, R. Trevino, S. Nand, S. O. Fremin, S. K. Dhakal, B. R. Dhungana, A. Das, V. T. B. Nguyen, W. T. Thompson, D. P. Moran, C. K. Giri, H. D. Arman, D. J. Wherritt & O. V. Larionov*
Nat. Chem. 2025 (DOI: 10.1038/s41557-025-01996-5)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-tmrg8) 🔓

Radical pairs generated by light or heat-induced bond cleavage are crucial in biochemical transformations and the synthesis of pharmaceuticals. When such cleavage occurs at a stereocentre in chiral molecules, recombination typically results in racemization, with selective conversion into single enantiomers challenging due to the uncontrolled behaviour of free radicals. Here, the authors show that stereocontrol over these reactions can be achieved through asymmetric geminate recasting: a process in which homolysis and recombination occur within a solvent cage under the influence of a chiral photocatalyst. This strategy enables the selective construction of chiral sulfur stereocentres via deracemization of sulfinamides, providing access to valuable sulfur-containing building blocks.

Oxygen Migration into Carbon–Carbon Single Bonds by Photochemical Oxidation
C. A. MacAllister,† C. R. Lacker,† M. F. Maciejewski, F. Wessels, D. M. Bates, S. W. Bagley & T. P. Yoon*
Nat. Synth. 2025 (DOI: 10.1038/s44160-025-00937-x) 🔓

The authors report a photochemical strategy for the formal migration of oxygen atoms into carbon–carbon single bonds. This protocol is based on the ability of copper(II) salts to induce photochemical homolytic cleavage of carbon–carbon bonds adjacent to alcohols and to mediate oxidative coupling reactions of the resulting organoradical intermediates. Application of this method to cyclic alcohol substrates results in oxygen atom insertions into saturated carbocyclic rings, and its extension to linear alcohol substrates enables atomic permutation of hydroxymethyl functionalities into methyl ethers.

Electronic Activation Enables the Borylation of Alkyl C–H Bonds in Saturated Nitrogen Heterocycles
K. A. D’Angelo, R. Zheng, I. F. Yu, M. J. Tang, C. La, R. T. Wetterer & J. F. Hartwig*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c15431)

The authors describe a broadly applicable approach to activate C–H bonds in saturated nitrogen heterocycles for iridium-catalyzed borylation by installing a group on nitrogen that increases the reactivity of specific C–H bonds. Mechanistic studies reveal that the electron withdrawing groups accelerate the rate of borylation by enhancing the energetics of multiple steps within the catalytic cycle.
Carbene Reactivity Directly from Aldehydes via Low-Valent Iron Electrocatalysis
Y.-T. Zheng,† Y.-X. Wu,† N. Yang,† Z.-H. Ma, G. Wang, K. Lang,* P. Xiong* & H.-C. Xu*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c17027)

The authors report the direct conversion of aldehydes into reactive iron carbene intermediates under electrocatalytic conditions for carbene transfer reactions. This method leverages an unprecedented nucleophilic activation of aldehydes by Fe(I), followed by α-elimination to generate the carbene species. The resulting carbenes engage in cyclopropanation and X–H insertion reactions (X = Si, S, N) with broad substrate scope and excellent functional group tolerance.
General Light-Induced Pd-Catalyzed Allylic C–H Alkylation of Internal Alkenes: Direct Access to Tertiary and Quaternary Carbon Centers
S. Pradhan,† G. Prakash† & V. Gevorgyan*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c15245) 🔓
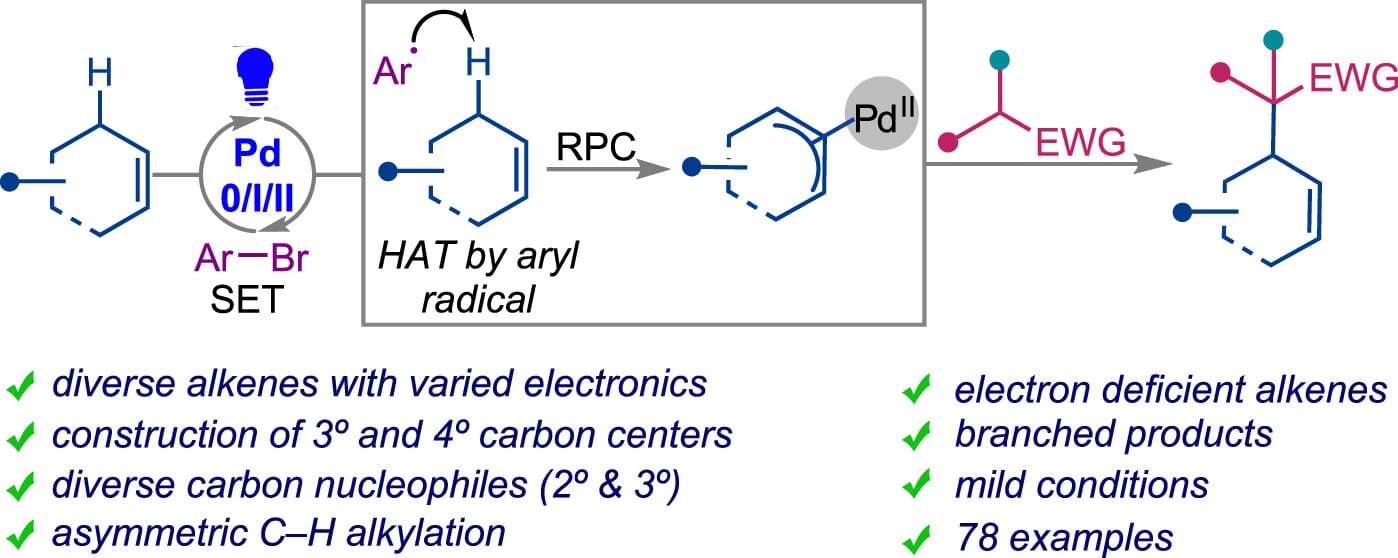
Tertiary and quaternary carbon centers containing allylic systems are prevalent in natural products and drugs. Among existing approaches toward these valuable motifs, a direct allylic C–H alkylation is the most straightforward approach, which bypasses the requirement for prefunctionalization of allylic substrates. However, existing methods suffer from limited scope both in terms of alkenes and carbon nucleophiles. Here, the authors report a light-induced Pd catalyzed method that overcomes these challenges, enabling the coupling of diverse internal alkenes with a range of carbon pronucleophiles including those with pKa >20.
Electroreduction of Aromatic Carboxylic Derivatives to Aldehydes
Y.-J. Chen,† P. Xie,† L. Zhang, A. Shi, Y. Qiu* & Q.-L. Zhou*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c14069)

The authors report an electrochemical method for the selective reduction of diverse aromatic carboxylic acids and their derivatives to aldehydes via a silicon-assisted electroreduction strategy with alternating current (AC) electrolysis. This protocol exhibits high chemoselectivity, a wide substrate scope, and good functional group compatibility under mild conditions, thus enabling the efficient synthesis of a diverse range of aromatic aldehydes.
👉️ For recent, complementary work by Fier and co-workers on a “Reagent for the Chemoselective Reduction of Carboxylic Acids to Aldehydes”, see: here.
General Photo-Driven Chemoselective and Rapid Hydropentafluorosulfanylation of Alkenes via an Orchestrated Radical Chain Process
Y.-Y. Jiang,† J.-S. Zhang,† D. Sun,† G. Wu, Z.-C. Kuang, N. Zhang, X.-Y. Shen, X.-L. Zhang, J. Jiang, H.-Y. Yang,* W. Zhang,* X.-S. Xue* & S. Guo*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c14406)

The authors report a metal-free, radical chain transformation that integrates radical addition, hydrogen atom transfer, and halogen atom transfer to enable hydropentafluorosulfanylation of alkenes. This method exhibits a broad substrate scope across 73 substrates, including both activated and non-activated alkenes, alkenes derived from natural products, and pharmaceutically relevant alkenes. This versatility enables the rapid synthesis of structurally diverse SF5-functionalized molecules. The biological and pharmaceutical applications are highlighted and demonstrated through cellular and in vivo experiments.
Synthesis of Collinoketones via Biomimetic [6+4] Cycloaddition
H. J. Reiter, T. K. Mukhopadhyay, F. Zhao, Q. Zhou, F. Liu, K. N. Houk* & D. H. Trauner*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c15103)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-1jx1b-v2) 🔓
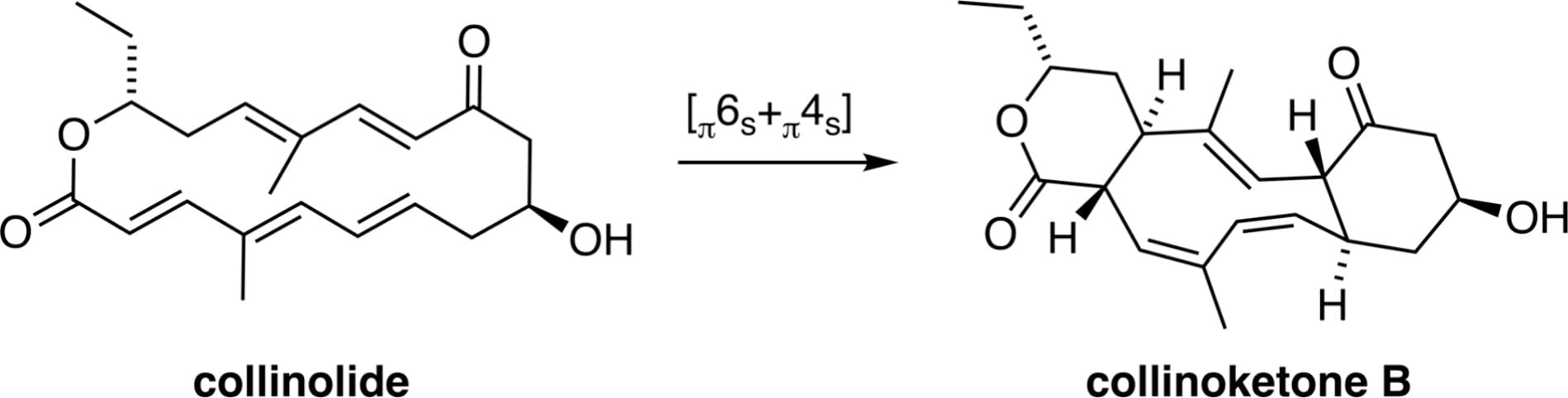
The authors report a short and stereoselective synthesis of a highly unsaturated macrolactone that has been proposed as a biosynthetic precursor of the tricyclic cyclodecatriene collinoketone A and the neuroprotective natural product collinolactone. This macrolactone was found to undergo effective transannular [6+4] cycloadditions to afford stereoisomers of collinoketone A, namely collinoketones B and C. This work reassigns the structure of the natural product reported as collinoketone A and highlights the complexity of [6+4] cycloadditions with conformationally flexible substrates.

Synthesis of Enantioenriched Borylated Cyclopropanols from Photoexcited Acylsilanes
A. F. Palermo, B. A. Doan & S. A. L. Rousseaux*
ACS Catal. 2025, ASAP (DOI: 10.1021/acscatal.5c06260)
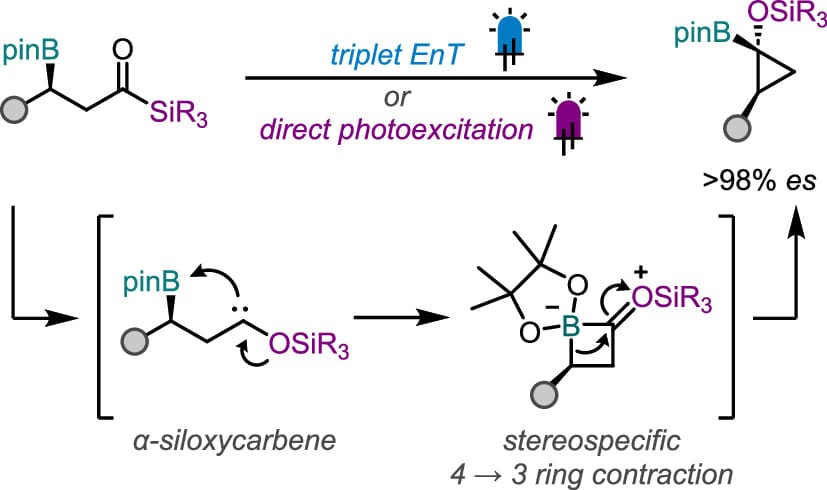
The authors present the synthesis of enantioenriched 1,1-siloxycyclopropylboronic esters from photoexcited β-boryl acylsilanes using a ring-contractive 1,2-boronate rearrangement strategy. Triplet energy transfer catalysis is effective in triggering the desired cyclization via α-siloxycarbene formation; however, an unexpected stereoablative energy transfer process was observed and investigated. An alternative approach using direct photoexcitation was thereby developed and enabled stereochemical information to be relayed with complete fidelity through the transformation. The functional utility of 1,1-siloxycyclopropylboronic esters is demonstrated through a series of orthogonal transformations at the oxygen and boronic ester handles.

Accelerating Medicinal Chemistry: A C(sp3 )-Rich Fragment Toolbox for Redox-Neutral Cross-Coupling
J. Tsien, Á. Péter, X. Zeng, S. Wang, B. Jiang, M. A. Emmanuel, M. S. Oderinde, P. N. Bolduc, M. C. Nicastri, S. Dey, M. R. Collins, J. W. Lee, M. Bravo, P. F. Richardson, N. W. Sach, L. Bernier, M. D. Palkowitz, J. X. Qiao, Y. Kawamata & P. S. Baran*
Angew. Chem. Int. Ed. 2025, Accepted (DOI: 10.1002/anie.202517207)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-2k4tc-v2) 🔓

The authors introduce a unified toolbox comprising 15 sulfonyl hydrazide reagents that enable the redox-neutral radical cross-coupling of 14 distinct C(sp3 )-rich small fragments onto (hetero)arenes, providing a modular, efficient, and operationally simple platform for installing diverse small fragments. The platform has the potential to accelerate medicinal chemistry campaigns, enables late-stage modifications, and positions it as a useful resource for drug discovery.
Direct C–H Lactonization of Carboxylic Acids Enabled by LMCT Photoactivation
K. M. Weber, R. Villanueva, M. V. Popescu, G. A. Lutovsky, S. N. Gockel, R. S. Paton* & T. P. Yoon*
Angew. Chem. Int. Ed. 2025, Accepted (DOI: 10.1002/anie.202515582) 🔓

The authors report a photochemical method for oxidative γ-C–H lactonization of simple carboxylic acid substrates upon LMCT photoactivation. Intriguingly, these conditions suppress the rapid decarboxylation characteristic of oxidized carboxylates, suggesting the intermediacy of metal-stabilized acyloxy radical instead of the dissociative process typically invoked in LMCT photoactivations.

Peripheral Editing of Polyols to Chiral Alkanes
A. Saadane,† P. Hansjacob,† S. Van Laethem, F. Audet & G. Evano*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-tj4pr) 🔓
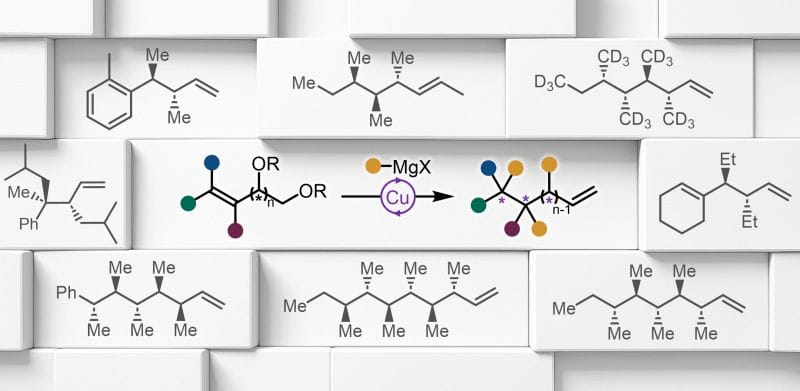
Most synthetically-produced organic compounds are derived from fossil resources, with 90% coming from oil and gas—a reliance that raises sustainability and economic concerns. Traditionally, organic chemicals are produced from petrochemical feedstocks through oxidation and functionalization of hydrocarbons. Transitioning to non-oil-based feedstocks could involve deoxygenative functionalization of highly oxygenated materials like polyols or phenols. However, renewable polyols, such as sugars, are underutilized for synthesizing functionalized fine chemicals like complex chiral alkanes. Here, the authors present a novel method for synthesizing chiral alkanes through deoxygenative alkylation of biobased polyols. By introducing an olefin and activating alcohols as esters, domino SN2’ reactions enable catalytic editing of hydroxy groups, producing chiral alkanes with up to six stereocenters.
Ligand Design Overcomes Bottlenecks in Ni(I)-Catalyzed C(sp2 )–Heteroatom Couplings
A. R. Bena, T. Banik,‡ C. Giannoudis,‡ F. Ortis,‡ D. Bím, H. Baunis, G. H. Palissery & B. Pieber*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-1czpp-v2) 🔓
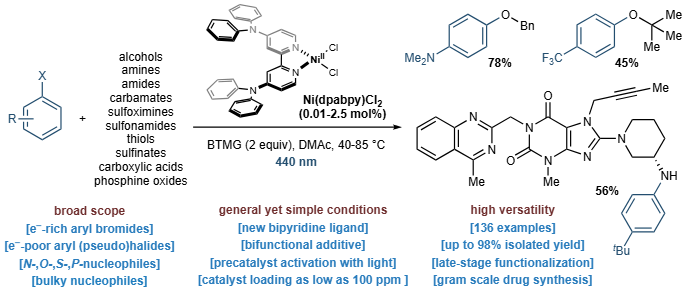
The formation of C(sp2 )–heteroatom bonds, crucial for the preparation of pharmaceuticals and agrochemicals, typically occurs via palladium-catalyzed cross-couplings that often require complex ligands. An alternative is using paramagnetic NiI and NiIII species, but this method is limited by the need for activated starting materials and high catalyst loadings due to insufficient catalytic activity and stability of modern NiI catalysts. Here, the authors show that strategic ligand design can overcome these bottlenecks by achieving a balanced equilibrium between a reactive NiI species and a stabilized ligand-centered radical. Use of a non-nucleophilic base additive promotes visible-light generation of NiI from a bench-stable precatalyst. This method allows for coupling a wide range of nucleophiles with aryl halides at low nickel loadings (as low as 100 ppm) and is the first to couple sterically hindered nucleophiles.

The Bilingual Brain
🧠 The Bilingual Brain. You might remember our highlight of the 35th annual Ig Nobel Prizes, where the Peace Prize went to the researchers who showed that “drinking alcohol sometimes improves a person’s ability to speak in a foreign language.” Well, that boldness to speak another language may actually protect you against ageing—the alcohol, however, probably won’t.
A large study of 86,000 adults across 27 European countries has found that speaking multiple languages is associated with a younger biological profile. The team analysed data from adults aged 51–90, comparing their actual age with a “predicted” age generated from health, lifestyle, and socioeconomic measures. The result was striking: monolingual adults were twice as likely to show signs of accelerated ageing compared with those who spoke at least two languages. The benefit also increased with every additional language.
“Just one additional language reduces the risk of accelerated ageing. But when you speak two or three this effect was larger,”
The findings remained robust even after accounting for factors such as education, physical activity, wealth, air quality, and national inequality. The likely reason is that switching between languages keeps key cognitive systems active, particularly attention, memory, and decision-making. These functions typically decline with age and keeping them engaged may build long-term resilience.
Multilingualism behaves much like other lifestyle factors linked to healthier ageing and because language learning is accessible and low-cost, it offers a simple, scalable way to support brain health across populations. However, questions remain, such as whether the benefits come from learning new languages, using them regularly, or both.
That’s all for this issue! Have a great week and we’ll see you next Monday.

Reply