- Synthesis Spotlight
- Posts
- Congratulations, It's Triplets
Congratulations, It's Triplets
💡 Alleged Data Fraud Derails $35 Million Alzheimer’s Trial

Monday 12th January – Sunday 18th January 2026 | Volume 3, Issue 2 |


Embracing Triplet Aryl Nitrenes for C-to-N Transmutation of Complex Molecules
D.-I. Park, Y. Gelato, J. M. Masterson, J. Ke, A. Tsymbal & M. D. Levin*
ChemRxiv 2026 (DOI: 10.26434/chemrxiv-2026-jn9zb) 🔓
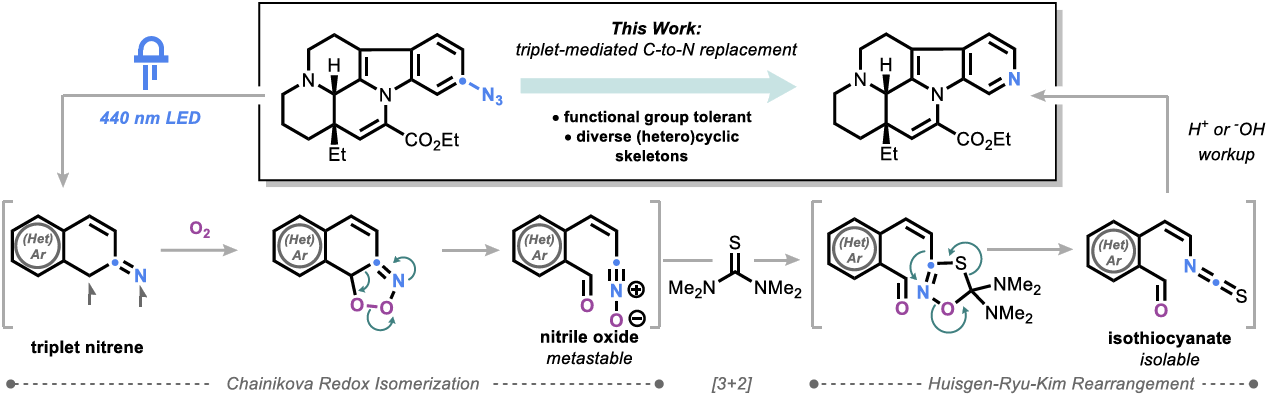
Despite significant proof-of-concept advances, aromatic C-to-N replacement reactions remain limitingly substrate-specific and exhibit an overreliance on harsh oxidation methods that preclude their deployment in demanding contexts. Transformations relying on singlet nitrene valence insertion, in particular, have suffered from parasitic intersystem crossing to the triplet spin state, which does not undergo the same rearrangement. Here, the authors report a reaction that productively engages triplet aryl nitrenes for C-to-N replacement with broad scope under mild conditions, enabling its application to complex, sensitive natural products.

The Photohydrolysis of Furans
N. Frank, M. B. Chaudhari, M. Leutzsch, B. Helmich-Paris, P. C. Bruzzese, D. Nater, N. Nöthling, A. Schnegg, S. R. Waldvogel & B. List*
Science 2026, 391, 267–274 (DOI: 10.1126/science.aec6532)

The defossilization of the chemical industry is accelerated by the shift from petroleum- to biomass-based feedstocks. At the center stage are bioderived furans, from which valuable platform chemicals can be obtained exclusively through oxidative or reductive processes. By contrast, the conceptually straightforward redox-neutral hydrolysis of furan to succinaldehyde and 2-substituted furans to 1,4-ketoaldehydes has been considered unfeasible owing to their endergonicity and polymerization side reactivity. In this work, the authors report the realization of this uphill furan hydrolysis through photocatalysis involving a highly strained, 10-membered 1,6-dioxecine intermediate. Succinaldehyde, as well as 1,4-ketoaldehydes, can be directly obtained from furans. Additionally, furfural derivatives undergo redox-enhanced Piancatelli rearrangements, accessing antimicrobial natural products (±)-Terrein and (±)-epi-Pentenomycins.

Catalytic Acyloin-type Heterocoupling of Thioesters via a Putative Cobalt Siloxycarbene
L. Kong,† K. Zong,† J. Guo & R. Shenvi*
Nat. Chem. 2026 (DOI: 10.1038/s41557-025-02036-y)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-hxpsz) 🔓

The authors report a method to access α-siloxycarbenes from thioesters via the reductive silylation of cobalt acyls. The reaction results in carbonyl dimerization with high hetero- and stereo-selectivity to yield unsymmetrical tetrasubstituted disiloxyalkenes, while avoiding competitive decarbonylation. These products can be further elaborated to new functionalized fragments, heterocycles and challenging enolsilanes.
Thermal [2+2] Cycloaddition as a Route to gem-Difluoro Heterobicyclo[n.1.1]alkanes
Y. Ning,† R. Wu,† Y. Ning,† Q. Song, J. Deng, Q. Jiao, P. Sivaguru, J. Mlynarski, G. de Ruiter, X. Hong* & X. Bi*
Nat. Chem. 2026 (DOI: 10.1038/s41557-025-02047-9)

The authors report a stepwise radical intramolecular thermal crossed [2+2] cycloaddition, enabled by the fluorine effect of in situ-generated N-(homo)allyl gem-difluoroenamines and homoallyl gem-difluorovinyl ethers. Silver-catalysed gem-difluoroalkenylation of N-(homo)allylamines and homoallyl alcohols with trifluoromethyl triftosylhydrazones, respectively, followed by an intramolecular crossed [2+2] cycloaddition of the in situ-generated gem-difluoroalkenes enables the synthesis of a range of medically relevant gem-difluoro heterobicyclo[n.1.1]alkanes, including azabicyclo[2.1.1]hexanes, azabicyclo[3.1.1]heptanes and oxabicyclo[3.1.1]heptanes.

Catalytic Asymmetric Hydroalkylation of 1,1-Dialkyl-Substituted Alkenes with Unactivated Alkyl Electrophiles
S. Ma,† L. Zhu,† J. Yin,† L. Wang, X. Yuan, S. Wang, D. Shi, Q. Zhang & T. Xiong*
Nat. Synth. 2026 (DOI: 10.1038/s44160-025-00971-9)

The authors present a cobalt-catalysed asymmetric radical hydroalkylation of 1,1-dialkyl-substituted alkenes with unactivated alkyl electrophiles, facilitating the formation of C(sp3 )–C(sp3 ) bonds with simultaneous construction of traditionally unaccessible fully alkyl-substituted chiral tertiary carbon centres attaching substituents possessing similar steric and electronic properties.

Electrochemical Indole Skeletal Editing via Single-Carbon Atom Insertion
Y. Chen,† G. Feng,† Y. Shen, Z. Liu, C. Sun, W. Ji,* H. Huang* & S. Pang*
J. Am. Chem. Soc. 2026, ASAP (DOI: 10.1021/jacs.5c19912)
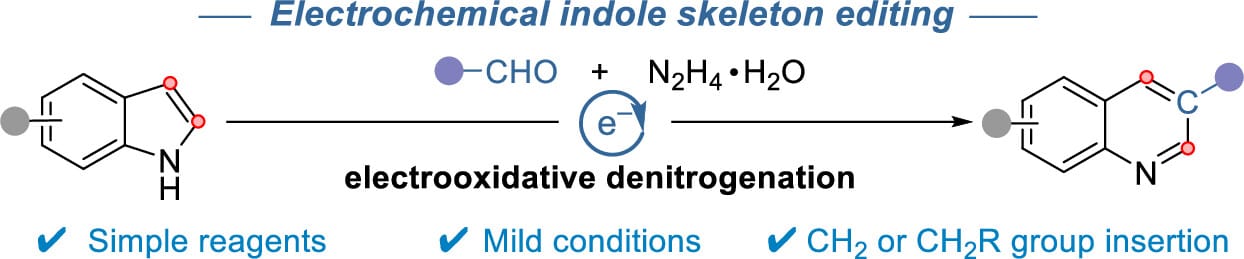
The authors report an electrochemical single-carbon insertion strategy for the skeletal editing of indoles into quinolines, which proceeds without the need for specialized reagents or transition-metal catalysts. The reaction is proposed to proceed via an electrooxidative denitrogenation process, in which in situ formed hydrazones generate reactive carbene intermediates that promote ring expansion.
Photolysis of CO2 Carbamate for Hydrocarboxylation Reactions
E. Azzi, M. Rodríguez-Martínez, S. R. N. Kolusu, J. Scarfiello, J. A. Varela & M. Nappi*
J. Am. Chem. Soc. 2026, ASAP (DOI: 10.1021/jacs.5c21208) 🔓

The authors present a new catalytic CO2 activation mode for hydrocarboxylation reactions. Key to this methodology is the formation of a CO2 carbamate with a phenothiazine catalyst, which sets the required trigonal geometry for the release of CO2•– via photolysis upon absorption of visible light. The polarity-reversed CO2•– is employed in the hydrocarboxylation reactions of alkenes and heterocycles. This protocol is distinguished by its mild reaction conditions, wide substrate scope and broad applicability, even in the context of pharmaceutical cores.
Enantioselective Radical Addition of Carboxylic Acids to Imines through Cooperative Copper/Acridine Catalysis
H. Huang & G. Xia*
J. Am. Chem. Soc. 2026, ASAP (DOI: 10.1021/jacs.5c22180)

The authors report a modular strategy for the asymmetric synthesis of chiral α-amino acids and amines through the decarboxylative radical addition to imines. This protocol leverages readily available, cost-effective carboxylic acids, aldehydes, and anilines as starting materials, enabled by the synergistic catalysis of acridine and copper. The reaction operates under ambient conditions, exhibits excellent functional group compatibility, and delivers high stereocontrol across a diverse substrate scope.

Enantioselective C–H Functionalization: Logic and Applications in the Total Synthesis of Natural Products
P.-F. Qian & B.-F. Shi*
Chem. Sci. 2026, Accepted (DOI: 10.1039/D5SC08653A) 🔓

The integration of enantioselective C–H functionalization into synthetic planning has emerged as a transformative strategy, offering streamlined and atom-economical routes to complex molecular architectures. This review summarizes the pivotal role of these methodologies in the asymmetric total synthesis of natural products. Recent breakthroughs have been organized according to three distinct C–H functionalization pathways: (i) radical hydrogen atom abstraction, (ii) metallocarbene C–H insertion, and (iii) C–H metalation.

Fused and Spirocyclic Sulfones for Medicinal Chemistry via [3+2] Cycloaddition of Thiocarbonyl Ylide
T. V. Rudenko, O. O. Stepaniuk, V. M. Timoshenko, A. A. Tolmachev, S. V. Shishkina & P. K. Mykhailiuk*
Org. Lett. 2026, ASAP (DOI: 10.1021/acs.orglett.5c05431)

The [3+2] cycloaddition between thiocarbonyl ylide CH2=S(+) –CH2(−) and electron-deficient cyclic alkenes provides bicyclic sulfides. The reaction efficiently works on milligram, gram, and even multigram quantities. Standard modifications of the obtained products give fused and spirocyclic sulfones for medicinal chemistry.
Direct Amidation of Tertiary N-Benzylamines
C. A. MacAllister, Y. Jiang & A. C. Sather*
Org. Lett. 2026, ASAP (DOI: 10.1021/acs.orglett.5c05384)

Protecting groups are standard tools for mitigating chemoselectivity challenges in complex molecule synthesis; however, the inefficiencies of protecting group manipulations and difficulties associated with their cleavage can be problematic. Leveraging routinely used protecting groups as handles for desired functionalization would eliminate these drawbacks while retaining suppression of undesired reactivity. Here, the authors achieve this goal by enabling the direct amidation of tertiary N-benzylamines under mild conditions, delivering medicinally important acrylamides and other amides with high yields and broad functional group tolerance. The method is readily implemented and demonstrated in the endgame synthesis of sensitive acrylamide Active Pharmaceutical Ingredients (APIs), including the KRASG12C inhibitor MK-1084.

Benzoxaborole and Beyond: The Emergence of Cyclic Hemiboronic Acids as a Versatile Chemotype in Medicine, Catalysis, and Materials
J. J. Blackner & D. G. Hall*
Chem. Rev. 2026, ASAP (DOI: 10.1021/acs.chemrev.5c00703)
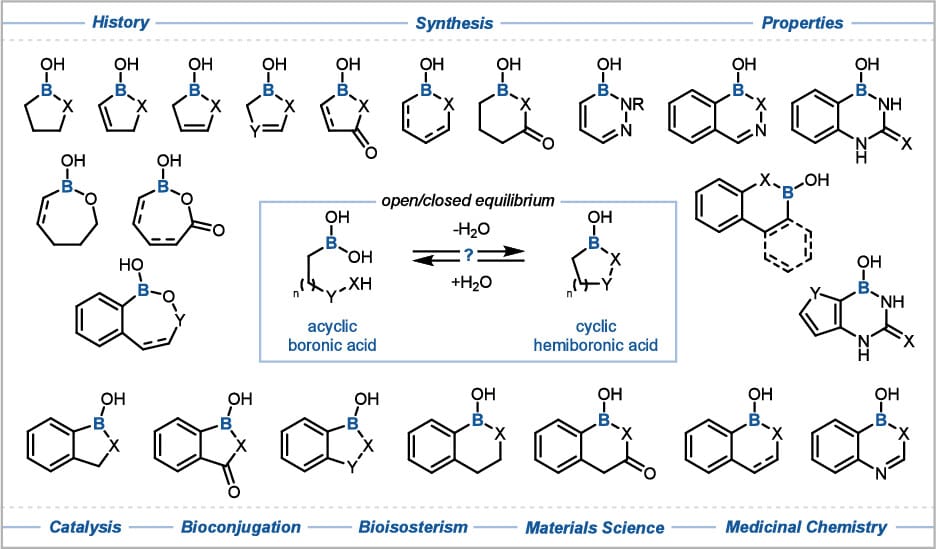
Cyclic hemiboronic acids are boron-containing heterocycles with one exocyclic boranol (B–OH) group, one endocyclic B–C bond, and one endocyclic B–heteroatom (O or N) bond. Unlike the more explored boronic acids, these compounds have gained interest due to the success of benzoxaboroles in drug discovery, highlighted by the approval of tavaborole and crisaborole. Over the past two decades, interest in nonaromatic and pseudoaromatic hemiboronic heterocycles has grown, with applications in organocatalysis, bioconjugation, drug discovery, natural product synthesis, and dynamic materials. This article reviews these compounds, discussing preparative methods, structural characteristics, and properties like open-closed equilibrium, acidity, and molecular interactions. Ring size and heteroatom type significantly affect acidity, reactivity, and aromatic character, influencing their applications.

Alzheimer’s Trial Collapses
🧠 Alzheimer’s Trial Collapses. A lawsuit by U.S. biotech firm T3D Therapeutics has raised serious concerns about the integrity of Alzheimer’s disease clinical trials, alleging that multiple South Florida research sites falsified data in a $35 million study of its experimental drug, T3D-959. T3D says the mid-stage trial initially appeared promising, suggesting cognitive benefits from the drug, which targets glucose metabolism in the brain. However, a subsequent review of patient-level data revealed what the company described as “medically impossible” results.
According to T3D, some placebo patients appeared to show cognitive improvement, many participants did not in fact have Alzheimer’s disease, and blood samples from supposed drug recipients showed no detectable trace of T3D-959. After discarding the suspect data, too few valid participants remained to support any conclusions, effectively nullifying the trial. T3D has accused five Miami-area trial sites and its contract research organization (CRO), Clinilabs, of data manipulation and of enrolling ineligible “professional patients” to boost revenue. These individuals are alleged to exaggerate or fabricate symptoms in order to enroll in multiple trials simultaneously and collect participation fees, sometimes without ever taking the drug at all. Clinilabs and the trial sites deny the allegations and argue that the trial failed because of poor study design.
South Florida has become a major hub for Alzheimer’s trials due to its large, diverse, and relatively elderly population, as well as a dense network of research clinics. However, several of the same sites named in T3D’s lawsuit have previously been cited for deficiencies in other studies, including enrolling patients who did not meet eligibility criteria. Experts say Alzheimer’s trials are particularly prone to abuse: symptoms can be faked, cognitive assessments have subjective elements, and financially motivated participants may attempt to join multiple studies at once. Similar problems have been reported by other drug developers. Annovis Bio and BioVie say Alzheimer’s trials drawing heavily from Miami-area sites were compromised by invalid patient data, setting their programs back by years.
While allegations of clinical trial misconduct are not uncommon, enforcement actions remain rare. Bioethicists argue that regulators should more aggressively sanction CROs and trial sites found to have falsified data, including barring them from future federally regulated research. Unreliable clinical data can be as damaging as an ineffective drug, erasing years of effort and investment while ultimately harming patients who urgently need effective therapies.
That’s all for this issue! Have a great week and we’ll see you next Monday.
Reply