- Synthesis Spotlight
- Posts
- CN You Believe It?
CN You Believe It?
💡 From COVID to Cancer: mRNA Vaccines Boost Survival Rates

Monday 20th October – Sunday 26th October 2025 | Volume 2, Issue 40 |


Direct Catalytic Deaminative Cyanation of Aliphatic Primary Amines
J.-H. Xue, S. Lin, J. Huang, T. Kang, B. Park, M. D. Levin,* M. Kim* & H. Wang*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-6bvbp) 🔓

The authors report the first catalytic, direct deaminative cyanation of aliphatic primary amines without preactivation. This method employs an anomeric amide for radical generation from amines and TMSCN as the cyano source, with a copper catalyst and N-fluorosulfonamide cooperatively facilitating both radical coupling and propagation. This work notably represents the first successful integration of anomeric amide activation with a transition metal-catalyzed bond-forming manifold. The strategy enables not only efficient cyanation but also asymmetric deaminative cyanation, stereocenter inversion, and downstream transformations such as homologation and CO insertion.

Selective Methylene Oxidation in α,β-Unsaturated Carbonyl Natural Products
C. Ahn, A. Gomez, M. A. Hartmann & M. C. White*
Nature 2025 (DOI: 10.1038/s41586-025-09742-0)

While catalytic systems have been developed for the selective oxidation of methylene C–H bonds in the presence aromatics and N-heterocycles, olefins remain an unsolved problem. Here, the authors show that by replacing the carboxylic acid with a H-bond donor solvent in sterically hindered manganese PDP catalysts, the active oxidant changes to one that accelerates electron rich methylene oxidation and significantly slows epoxidation of electron deficient olefins (kC-H[O]/kepox = 38.5). Chemoselective methylene oxidation is demonstrated in forty-five molecules housing α,β-unsaturated carbonyls where all previous methods afforded allylic oxidation or epoxidation.

Hydrogen-Mediated Reductive Cross-Coupling of Aryl Iodides with Activated Aryl and Heteroaryl Bromides
W. Shen,† Y.-H. Chang,† B. I. Gonzalez, A. P. Looby, J. J. Dotson,* N. A. White,* B. P. Carrow* & M. J. Krische*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c15725)
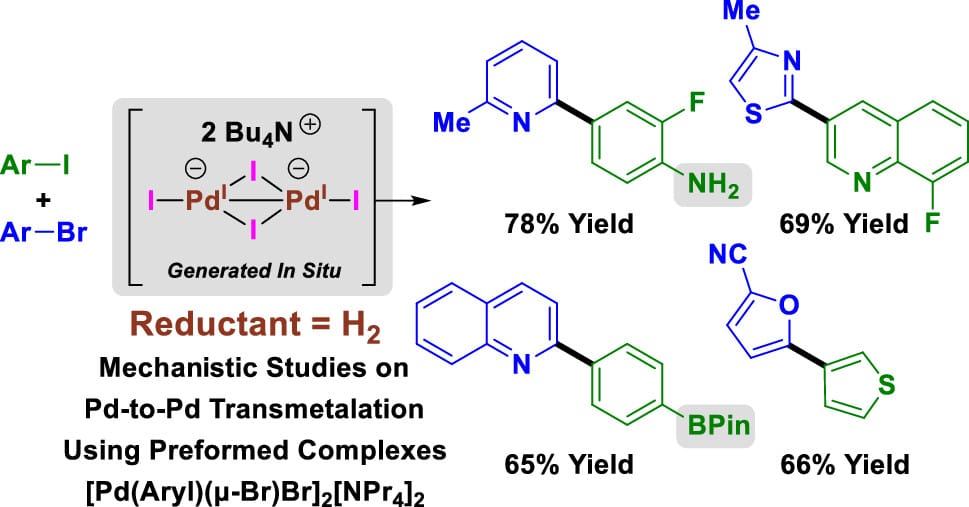
Hydrogen-mediated reductive cross-couplings of aryl iodides with activated aryl and heteroaryl bromides are described, along with related homocouplings (Ullmann reactions) and vinylic reductive couplings that occur with cine-substitution. To corroborate key events in the catalytic cycle, Pd-to-Pd transmetalation and cross-selective reductive elimination, dianionic diarylpalladate complexes [Pd(Aryl)(μ-Br)Br]2[NPr4]2 were prepared. Stoichiometric reactions of these complexes in the presence of iodide demonstrate that Pd-to-Pd transmetalation by way of monomeric arylpalladates occurs at a greater rate than reductive elimination and that cross-selective C–C reductive elimination is favored for electronically distinct aryl partners.
Photocatalyst-Regulated Trifluoromethoxylation of Aryl Halides under Silver Promotion
J. Zhou, L. Chen, Z. Miao, J. Li, Y.-X. Luan,* L. Chen* & P. Tang*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c11167)

The authors report the first trifluoromethoxylation of aryl halides, enabled by a photocatalytic strategy via aryl cationic radical intermediates. A key advancement is the use of silver salts, which unlocks the participation of electronically neutral and electron-withdrawing aryl halides. Moreover, this method demonstrates exceptional chemoselectivity for aryl chlorides and bromides.
Deep-Red to Near-Infrared Light-Driven Radical Generation from Organoboron Compounds via Ligand-Induced Direct Excitation Catalysis
Y. Miyamoto, K. Muraoka, S. Murakami,* T. Matsudaira & H. Ohmiya*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c17266)
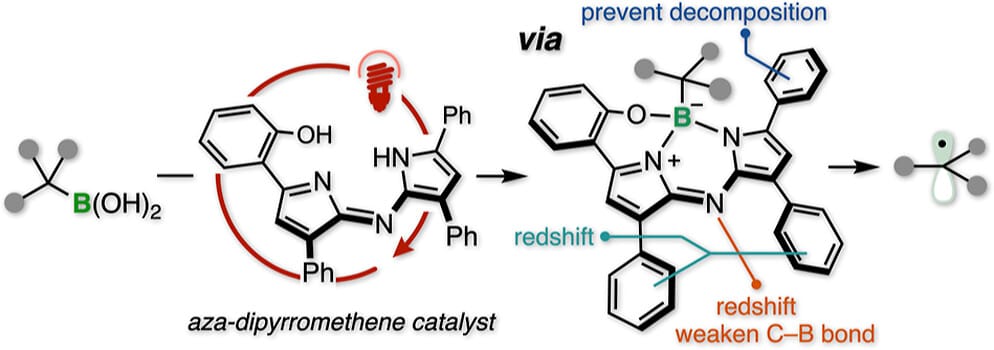
The authors report a catalytic strategy for generating carbon-centered radicals from organoboron compounds under deep-red to near-infrared (DR to NIR) light irradiation via direct excitation of substrate–catalyst complexes. Aza-dipyrromethene (ADP) catalysts form photoactive borate intermediates that enable C–B bond cleavage through ligand-induced photochemical activation. This method allows diverse transformations, including Giese addition, C-heteroatom bond formation, radical–radical coupling, and nickel-catalyzed cross-coupling reactions.
Catalytic Enantioselective Diels–Alder Reaction of Dienes with Acyclic and α,β- and β,β-Disubstituted Enones
J. Samsonowicz-Górski, S. Ghosh, N. Tsuji, M. Leutzsch, G. Breitenbruch, N. Nöthling, P. Kraft & B. List*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c13725) 🔓
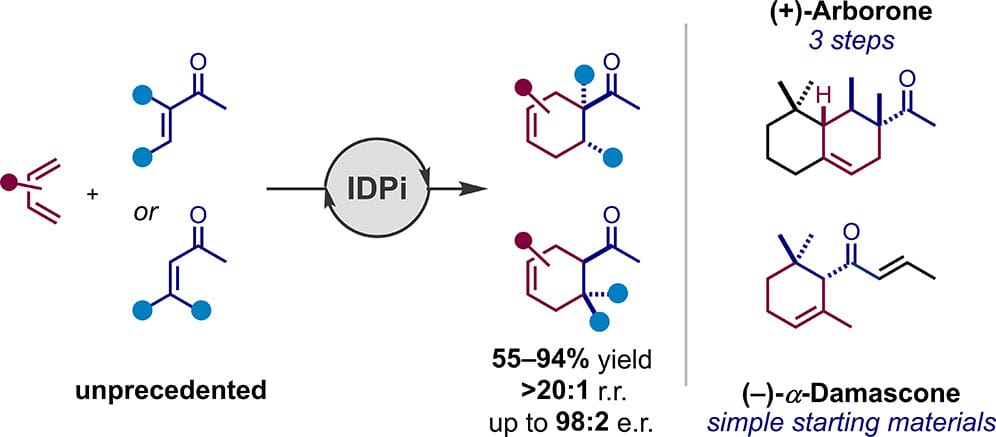
Despite the wealth of methods to enantioselectively catalyze Diels–Alder reactions of α,β-unsaturated carbonyl dienophiles, until now, not a single example with an acyclic enone that is either β,β- or α,β-disubstituted has been reported. Here, the authors disclose a general Brønsted acid-catalyzed enantioselective Diels–Alder reaction of various aliphatic acyclic disubstituted enones with hydrocarbon dienes featuring diverse steric and electronic properties. The method has been employed to synthesize several enantiopure targets, including well-known fragrance components such as arborone and the damascones.
Azaanthraquinone PCET Catalysis Enables Chemoselective Decarboxylative Functionalization of Diverse Carboxylic Acids
T. Inoue, D. Tomiya, M. Fuki, Y. Kobori, M. Higashi, K. Uesaka, A. Yamakata, S. A. Kawashima, K. Yamatsugu, H. Mitsunuma* & M. Kanai*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c10807)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-k80kg) 🔓

In this study, a novel proton-coupled electron transfer (PCET) catalyst containing an azaanthraquinone skeleton has been developed for the decarboxylative functionalization of a wide range of carboxylic acids, including aromatic and perfluoroalkyl carboxylic acids under mild conditions with high chemoselectivity. By integrating the aza-arene and benzoquinone moieties into a single molecule, this catalyst suppresses the previously problematic back electron transfer through rapid intersystem crossing from singlet to triplet states and the facilitated electron transfer from the substrate to the excited triplet catalyst via PCET.
Transition-Metal-Free Site-Selective β- and γ-C–H Borylation of Aliphatic Amines
S. Sarkar,† N. K. Deo,† T. Zhang & V. Gevorgyan*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c12618)
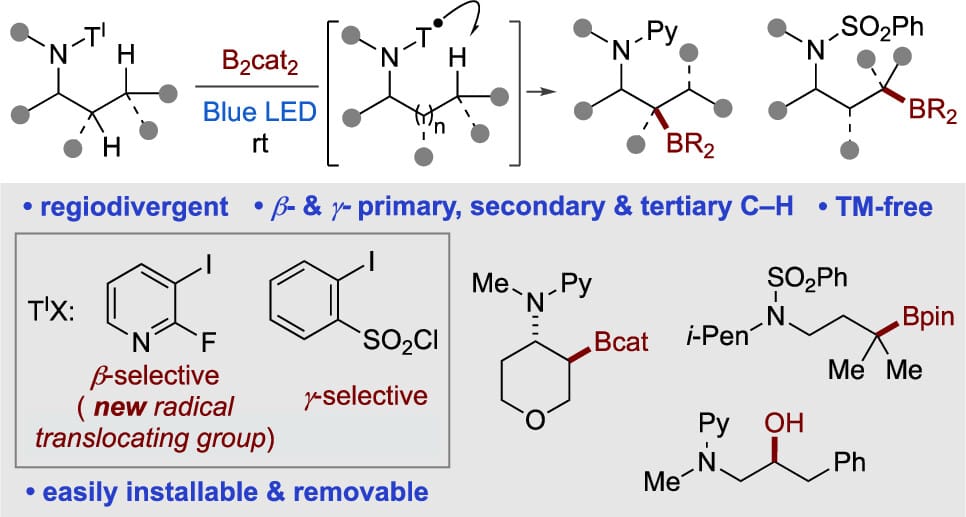
A mild photoinduced transition metal-free method for β- and γ-C(sp3 )–H borylation of amines has been developed. This protocol features a regioselective intramolecular hydrogen atom transfer (HAT) process to access key amino alkyl radical intermediate employing commercially available, installable/removable iodoaryl tethers. The method allows for the activation of primary, secondary, and tertiary C–H sites of a broad range of acyclic and cyclic secondary amines and the utility of this protocol has been demonstrated through formal C–H oxidation reaction and other late-stage derivatizations.
Divergent Total Synthesis of Pleurotinoid Natural Products
J.-B. Qiao,† J.-Y. Pei,† Y. Zhang, Q.-W. Zhang & Y.-M. Zhao*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c14395)
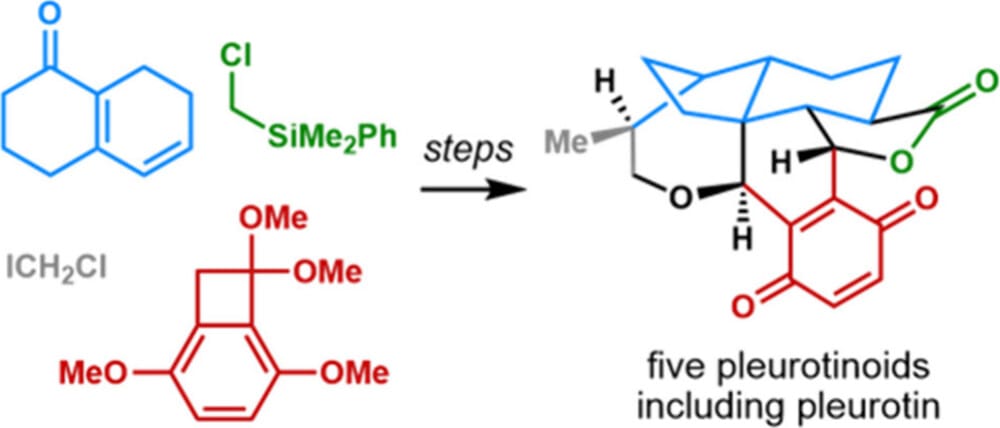
The authors report a new strategy for the divergent and efficient synthesis of pleurotinoids, featuring an intermolecular 1,6-addition/vinylogous Mukaiyama aldol reaction sequence, a rare 4π-electrocyclic ring-opening/vinylogous Michael cascade reaction, diastereoselective Wolff rearrangement ring contraction, facial-selective reduction of terminal allylic alcohols, and late-stage oxidative cyclization.
Concise Enantioselective Total Synthesis of (+)-Punctaporonin U
P. Gri, Q. Wang & J. Zhu*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c15423)
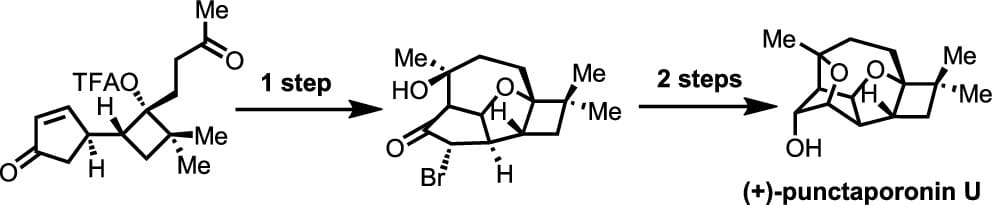
The authors report the first enantioselective total synthesis of (+)-punctaporonin U, a cage-like pentacyclic sesquiterpene bearing eight contiguous stereocenters. The synthesis features three key transformations: i) a rare cis-selective Mukaiyama-Michael addition, ii) a domino oxa-Michael addition/aldol reaction/bromination sequence that constructs both a fused five-membered ring and a 1,4-bridged 7-membered ring, and iii) an intramolecular SN2 displacement to install the 1,3-bridged tetrahydrofuran ring.
A Reductive Mechanochemical Approach Enabling Direct Upcycling of Fluoride from Polytetrafluoroethylene (PTFE) into Fine Chemicals
M. E. Lowe, B. M. Gallant, N. Davison, M. N. Hopkinson, D. J. Kubicki,* E. Lu* & R. J. Armstrong*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c14052) 🔓
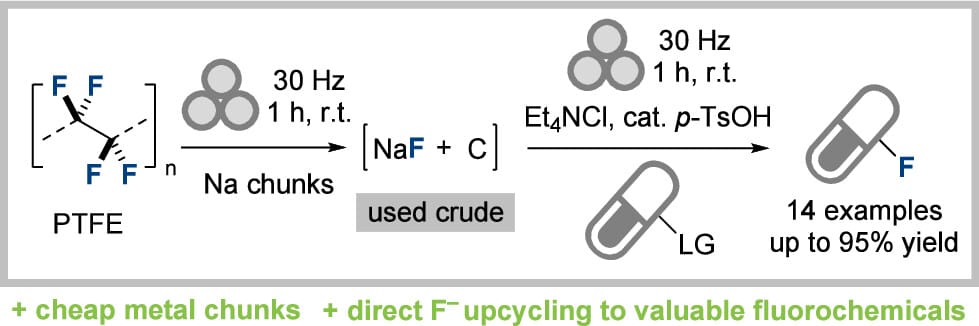
The authors report a straightforward mechanochemical approach for the reductive defluorination of polytetrafluoroethylene (PTFE) generating an environmentally benign mixture of elemental carbon and sodium fluoride. The process employs cheap and readily available chunks of sodium metal, proceeding rapidly at room temperature, in the absence of any organic solvent to form sodium fluoride (NaF) in 98% yield. The fluoride generated in the process can be directly upcycled into fine chemicals through in situ mechanochemical fluorination reactions, delivering valuable sulfonyl fluoride and acyl fluoride products in excellent yields.

Stereoselective Synthesis of Alkenyl Fluorides and Alkynes by Defluoro Coupling of Trifluoromethyl Arenes
J. L. Rosario-Collazo,† C. Westlund,† H. Keita & S. J. Meek*
Angew. Chem. Int. Ed. 2025, Early View (DOI: 10.1002/anie.202515710) 🔓

The authors report a practical, stereoselective method for synthesizing tri- and tetra-substituted 1,2-fluoro-borylalkenes and alkynes via defluorinative C–C coupling of CF3–arenes with organodiboron reagents. Enabled by a Lewis base activator, products are formed in up to 94% yield and >98:2 Z/E selectivity, with the utility of this method showcased through several product transformations.
Facile (Z)-Selective Synthesis of β,γ-Unsaturated Ketones by a Silicon-based Olefination Strategy
D. Aynetdinova,† J. Brześkiewicz,† N. Skoulikas† & N. Maulide*
Angew. Chem. Int. Ed. 2025, Early View (DOI: 10.1002/anie.202517069) 🔓

A silicon-based olefination strategy has been developed to access challenging (Z)-configured β,γ-unsaturated ketones from readily accessible branched vinyl silanes and acid chlorides. This method enables the straightforward synthesis of a broad variety of deconjugated ketones with high (Z)-selectivity under mild conditions and provides a new retrosynthetic disconnection to streamline the synthesis of complex scaffolds and natural products.

Cross-Electrophile Coupling of NHP Esters With Aryl Bromides Using an Inner-Sphere, Homogeneous Reductant
K. Ganguli, A. R. Cruz, J. B. Diccianni, P. García-Reynaga* & D. J. Weix*
ChemRxiv 2025 (DOI: 10.26434/chemrxiv-2025-60n8j) 🔓

Cross-electrophile coupling of aryl bromides with N-hydroxyphthalimide (NHP) esters offers a valuable strategy for forming C(sp2 )–C(sp3 ) bonds. However, developing a broadly applicable method remains challenging, particularly with electron-rich aryl bromides, often requiring electrochemical tools or electron-rich NHP derivatives. Here, the authors report new conditions that help overcome these limitations and provide a broad scope of reactivity without the need for specialized hardware. Key to success is the identification of a new general class of homogeneous reductants for XEC, 1,4-bis(trialkylsilyl)dihydropiperazines (Si-DHP).

Reagent for the Chemoselective Reduction of Carboxylic Acids to Aldehydes
E. Fung, K. M. Maloney & P. S. Fier*
Org. Lett. 2025, ASAP (DOI: 10.1021/acs.orglett.5c04063)
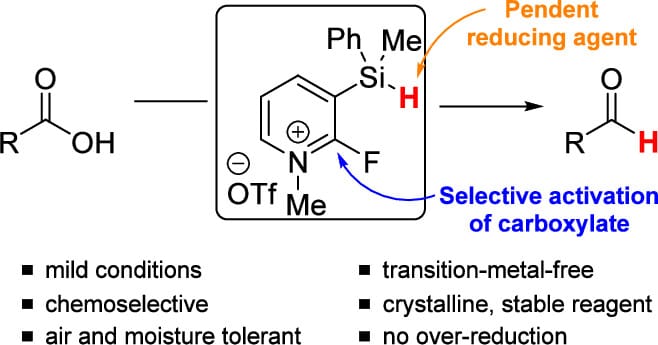
The authors report the development of a multifunctional reagent for the direct conversion of carboxylic acids to aldehydes under mild conditions and with exquisite chemoselectivity. These reactions proceed in aqueous solvent at room temperature in 20 min, can be done open to air, require no metal catalysts, and employ near-stoichiometric amounts of both reducing agent and a mild base. No over-reduction, epimerization, or reduction of other reducible functional groups is observed. With this reagent, a broad scope of complex, drug-like aryl and alkyl carboxylic acids can be reduced directly to aldehydes.

The results are in.
Thanks to everyone who voted! The overwhelming majority (85%) prefer receiving the newsletter on Mondays, so we’ll continue as usual. For those who prefer a different day of the week, we’ll look into whether it’s possible to accommodate this for specific readers. Thank you all very much for your feedback!


From COVID to Cancer
💉 From COVID to Cancer. In yet another blow to the anti-vaccine movement, the mRNA vaccines that changed the course of the pandemic and saved millions of lives, may now be helping cancer patients live longer.
Researchers at the University of Texas MD Anderson Cancer Center have found that people with melanoma or non-small cell lung cancer who received an mRNA COVID-19 vaccine within 100 days of starting immunotherapy with immune checkpoint inhibitors (ICIs) survived significantly longer than those who didn’t. For lung cancer patients, median survival nearly doubled—from 21 months to 37 months—while vaccinated melanoma patients lived so long that the study ended before an average could even be calculated.
The effect wasn’t seen with traditional flu or pneumonia vaccines, suggesting that the mRNA platform itself is key. Follow-up experiments in mice may indicate why: mRNA vaccines boost the immune system in cancer patients by triggering a surge of type I interferons, which activate immune cells to recognise and attack tumours. When paired with ICIs, this amplified immunovigilance translates into a markedly stronger anti-tumour response.
A clinical trial is already being planned to confirm the findings. Yet the work comes amid political backlash in the US, where roughly $500 million in federal funding for mRNA research has recently been cut.
That’s all for this issue! Have a great week and we’ll see you next Monday.

Reply