- Synthesis Spotlight
- Posts
- Amines → Whatever You Want Really
Amines → Whatever You Want Really
💡 Curiosity May Have Killed the Cat but It Gave Us MRIs & Ozempic

Note: Some weeks may be longer than others, but this week’s issue will be slightly shorter, focusing on eight standout papers that caught our attention.
Monday 27th October – Sunday 2nd November 2025 | Volume 2, Issue 41 |


Direct Deaminative Functionalization with N-Nitroamines
G. Tu,† K. Xiao,† X. Chen,† H. Xu,† H. Zeng, F. Zhang, X. Xue* & X. Zhang*
Nature 2025 (DOI: 10.1038/s41586-025-09791-5)

The authors report a direct deaminative strategy through the formation of N-nitroamines, enabling the direct conversion of inert aromatic C−N bonds into an array of functional groups: C−X, C−F, C−N, C−S, C−Se, C−O and C−C bonds. This operationally simple protocol establishes a unified strategy for one-pot deaminative cross-couplings by integrating deaminative functionalization with transition-metal catalyzed arylation, thereby streamlining synthesis and late-stage functionalization. The advantage of this transformation over other deaminative functionalization methods lies in its versatility across nearly all classes of medicinally relevant heteroaromatic amines, as well as electronically and structurally diverse aniline derivatives.

Bismuth-Photocatalysed Heck-type Coupling with Alkyl and Aryl Electrophiles
S. Ni, A. Stamoulis, V. A. Béland & J. Cornella*
Nat. Catal. 2025 (DOI: 10.1038/s41929-025-01438-y) 🔓

The authors report a conceptually distinct Heck-type coupling strategy that replaces transition metals with a photoactive bismuth complex, marking an advance in main group catalysis. The bismuth catalyst undergoes a photo-induced ligand-to-metal charge transfer processes, unmasking a Bi(II) species capable of halogen atom transfer processes with alkyl iodides. The multifaceted redox-dependent photophysical properties of the bismuth catalyst facilitate the coupling of aryl and alkyl electrophiles with styrenes through an intricate interplay of mechanistic steps.

Functional Group Transposition Enabled by Palladium and Photo Dual Catalysis
M. Xu,† C. Wu† & M. Chen*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c11429) 🔓
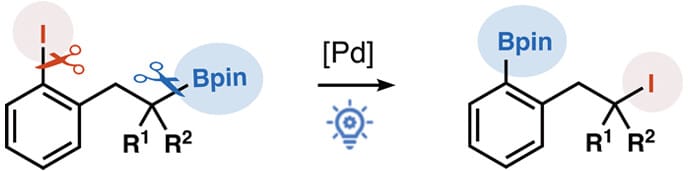
Functional group transposition has recently emerged as a powerful strategy to edit molecules and allow for access to novel chemical entities without significantly altering synthetic routes. Here, the authors disclose an unusual functional group transposition reaction. By using palladium and photo dual catalysis, this radical-induced process enables the transposition between an iodo group and a boryl group to convert iodoarenes appended with an alkylboronic ester to arylboronic esters appended with an alkyl iodide.
Pyridine-to-Pyridazine Skeletal Editing
W. Choi, A. Jang & S. Hong*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c15601)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-1jc74) 🔓

The authors report a skeletal editing strategy that converts pyridines into pyridazines by replacing one ring carbon with nitrogen while preserving aromaticity. The sequence comprises N-amine assembly, followed by an m-CPBA-mediated ring-remodeling sequence proceeding via a 1,2-diazatriene intermediate to effect carbon-to-nitrogen substitution. The two-step process is operationally simple, runs at ambient temperature in air, and requires no UV irradiation or preinstalled groups. The method shows broad functional-group tolerance, including complex, drug-derived molecules, providing rapid, scalable access to pyridazines.
Cyclopropylmethyl Boronic Esters as General Reagents in Transition-Metal Catalyzed Homoallylation Reactions
B. Lozano,† J. Teresa,† I. Fernández & M. Tortosa*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c15781) 🔓

The authors disclose the use of cyclopropylmethyl boronates as general reagents in Negishi-type homoallylation reactions. This strategy provides a novel approach to generate enantioenriched homoallyl-Zn species through boron-to-zinc transmetalation. Subsequent sp2 –sp3 cross-coupling offers a platform for the preparation of arenes, ketones, and 1,5-dienes containing a chiral homoallylic scaffold. The method has been applied to the late-stage functionalization of known drugs and the preparation of precursors of biologically relevant compounds.
From Alkylarenes to α-Amino Acid Derivatives via C-Difunctionalization
X. Wang, J. Liu, Z. Cheng, T. Wang, J. Peng, J. Meng, Y. Huang, Q. Sun* & N. Jiao*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c14247)

The authors report a novel C-difunctionalization strategy of alkylarenes via C–C bond cleavage, providing a unique pathway for the synthesis of unnatural amino acids. The success of this approach hinges on an entropy-driven remodeling strategy for skeleton restructuring and precisely controlled chemoselectivity for stepwise carbon editing.
One-Electron Approach for trans-Selective Alkyne Semi-Reduction via Cobalt Catalysis
R. Mondal,† L. Galmidi,† A. Tzaguy, T. Sason, M. Feller, M. A. Iron, L. Avram, R. Neumann & S. Gnaim*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c07630) 🔓

The authors introduce a new approach for the trans-semireduction of internal alkynes, enabled by a cobalt-catalyzed electrochemical radical pathway. This method offers a broad substrate scope, accommodating alkynes with diverse electronic and steric profiles, and displays high chemoselectivity and functional group tolerance. The methodology was extended to isotopically labeled trans-deuteration and demonstrated excellent chemoselectivity in substrates containing multiple alkyne motifs. Finally, mechanistic studies were performed, which helped guide the development of a complementary chemical oxidative protocol.
Kinetic, Spectroscopic, and Computational Investigation of Oxidative Aminative Alkene Cleavage Reveals an N-Iodonium-Iminoiodinane Pathway
F. Ruepp, V. Grebennikov, M. Avramenko, M.-O. Ebert & B. Morandi*
J. Am. Chem. Soc. 2025, ASAP (DOI: 10.1021/jacs.5c14977)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-6cdgb) 🔓
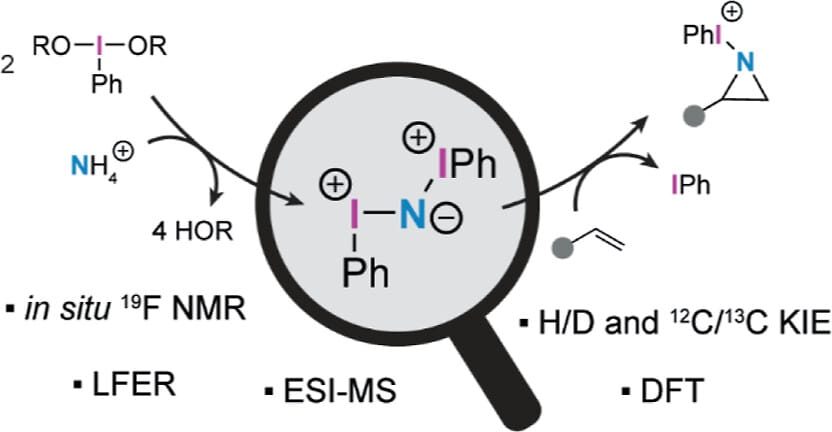
The authors present an extensive mechanistic study of a recently published oxidative aminative cleavage of alkenes, obtaining key insights into the understudied aspects of hypervalent iodine-mediated nitrogen atom insertion. Through these studies, the formation of an N-iodonium-iminoiodinane was shown to be rate-determining in this reaction. This species is highly electrophilic and capable of concerted, asynchronous transfer of a [PhI–N]+ unit to double bonds. These findings point toward the N-iodonium-iminoiodinane, not an iodonitrene, being the active N-atom transfer agent generated from the combination of hypervalent iodine(III) oxidants and ammonia.

Blue-Sky Research, Ground-Breaking Results
🌤️ Blue-Sky Research, Ground-Breaking Results. With research budgets tightening and funders seeking quick returns—or anything with “AI” in the title—it’s easy to forget that many of the world’s most transformative discoveries began as curiosity-driven research with no obvious applications. This week, Nature highlights seven such breakthroughs, a timely reminder that “blue-sky” research often yields the brightest outcomes.
Take PCR: born from studies of bacteria in Yellowstone’s hot springs, it has since become central to DNA fingerprinting, organ-donor matching, and COVID-19 testing. MRI grew out of fundamental research into atomic nuclei, while the path to Ozempic started with venomous lizards—Gila monsters. Even flat-screen TVs owe their existence to 19th-century chemists heating up carrot extracts. These advances weren’t driven by profit motives, they were simple rewards of letting science ask its own questions.
And yet, the funding that makes such discoveries possible is now under threat. Under President Trump, the NSF has terminated around a billion dollars’ worth of fundamental research (over 1,400 grants), while the NIH has cancelled billions more. The proposed 2026 budget would also gut non-defence R&D by 36%. At a time when we face major challenges from climate change to future pandemics, public investment in basic research remains essential if we are to achieve the next PCR, MRI, or CRISPR.
That’s all for this issue! Have a great week and we’ll see you next Monday.

Reply