- Synthesis Spotlight
- Posts
- 2025 Wrapped
2025 Wrapped
💡 The Best Science Images of 2025

Welcome to a special “2025 Wrapped” edition of Synthesis Spotlight.
As 2025 comes to a close, we’ve crunched the numbers to identify the Top 10 Most Popular Papers featured in the newsletter over the past 12 months. Spanning Science, Nature, Nature Chemistry and Angewandte Chemie, this list highlights where organic chemistry captured the most attention—from radical-enabled bond construction and deaminative cross-couplings to atom swaps, homologations, and ambitious total synthesis. This years themes and trends are:
Amines as privileged feedstocks: Deaminative chemistry dominated the year. Four of the top ten papers (#4, #5, #7, #9) transform amines—traditionally synthetic endpoints—into versatile radical precursors or coupling handles, cementing C–N bond cleavage as a mainstream tactic, particularly for late-stage diversification.
Radical cross-coupling: Radical chemistry underpins much of the list, with MacMillan’s “dynamic orbital selection” (#1), Leonori’s boryl radical platform (#7) and Baran’s redox-neutral cross-couplings (#8).
Reimagining functional group interconversions: Dong’s carbonyl-to-sulfur atom swap (#2) and Gaunt’s one-carbon alkene homologation (#6) underscore growing interest in skeletal editing.
Total synthesis still matters: Rounding out the list, Gaich’s total synthesis of (−)-spiroaspertrione A (#10) shows that complex molecule synthesis remains a cornerstone of the field, driving innovation and access to biologically relevant compounds.
Wednesday 1st January – Wednesday 17th December 2025 | 2025 Wrapped |


#1
Generalizing Arene C–H Alkylations by Radical–Radical Cross-Coupling
J. Großkopf, C. Gopatta, R. T. Martin, A. Haseloer & D. W. C. MacMillan*
Nature 2025, 641, 112–121 (DOI: 10.1038/s41586-025-08887-2)

The authors report the application of a new strategy for the selective coupling of differently hybridized radical species, termed “dynamic orbital selection”. This mechanistic model overcomes common limitations of Friedel–Crafts alkylations via the in situ formation of two distinct radical species, which are subsequently differentiated by a copper-based catalyst on the basis of their respective binding properties. As a result, a general and highly modular reaction is demonstrated for the direct alkylation of native arene C–H bonds using abundant and benign alcohols and carboxylic acids as the alkylating agents.
#2
Carbonyl-to-Sulfur Swap Enabled by Sequential Double Carbon–Carbon Bond Activation
Z. Zhang & G. Dong*
Science 2025, 388, 1436–1440 (DOI: 10.1126/science.adx2723)
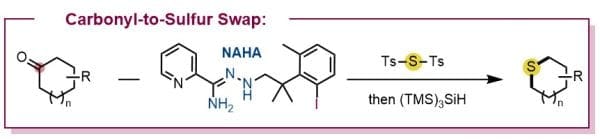
The authors report a two-step carbonyl-to-sulfur (CO-to-S) atom swap approach, enabled by a rationally designed N′-alkyl-hydrazonamide (NAHA) reagent that promotes forming pre-aromatic intermediates twice sequentially by different mechanisms, thereby achieving homolytic cleavage of both α-C−C bonds of the ketone substrates. A Ts−S−Ts (Ts, p-toluenesulfonyl) reagent mediates this process through successive intermolecular and intramolecular alkyl radical trapping by the central sulfur. This method shows a broad substrate scope and excellent chemoselectivity, providing a streamlined route to sulfur-containing scaffolds from readily available ketones.
#3
Myriad Aryne Derivatives from Carboxylic Acids
C. M. Seong,† S. S. Kargbo,† C.-L. Yu,† D. Gibney, J.-N. Boyn* & C. C. Roberts*
Nature 2025 (DOI: 10.1038/s41586-025-09830-1)
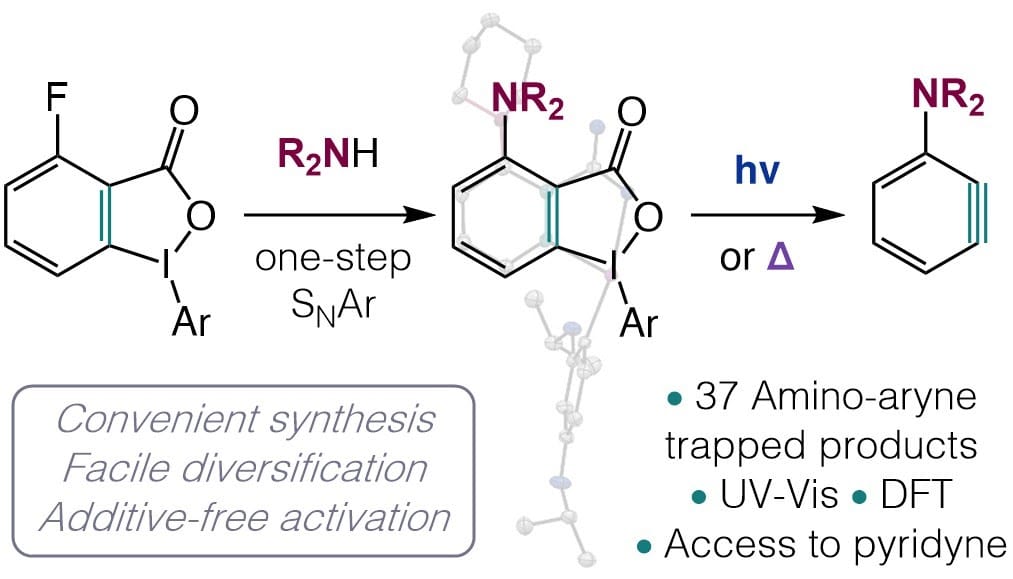
Aryne intermediates have significant synthetic potential, reacting with nucleophiles, participating in pericyclic reactions, and activating inert σ-bonds. However, their use is limited to niche applications due to the undesirable methods required for their generation. Here, the authors report the design of an aryne precursor to overcome this prohibitive barrier. Readily available carboxylic acids are derivatized in a single step to a make a precursor which is then activated by blue light or by heat. Dozens of previously unknown aminated arynes, including pyridynes, are generated in this work, opening the door to drug discovery using aryne intermediates.
#4
Direct Deaminative Functionalization with N-Nitroamines
G. Tu,† K. Xiao,† X. Chen,† H. Xu,† H. Zeng, F. Zhang, X.-S. Xue* & X. Zhang*
Nature 2025, 648, 341–348 (DOI: 10.1038/s41586-025-09791-5) 🔓
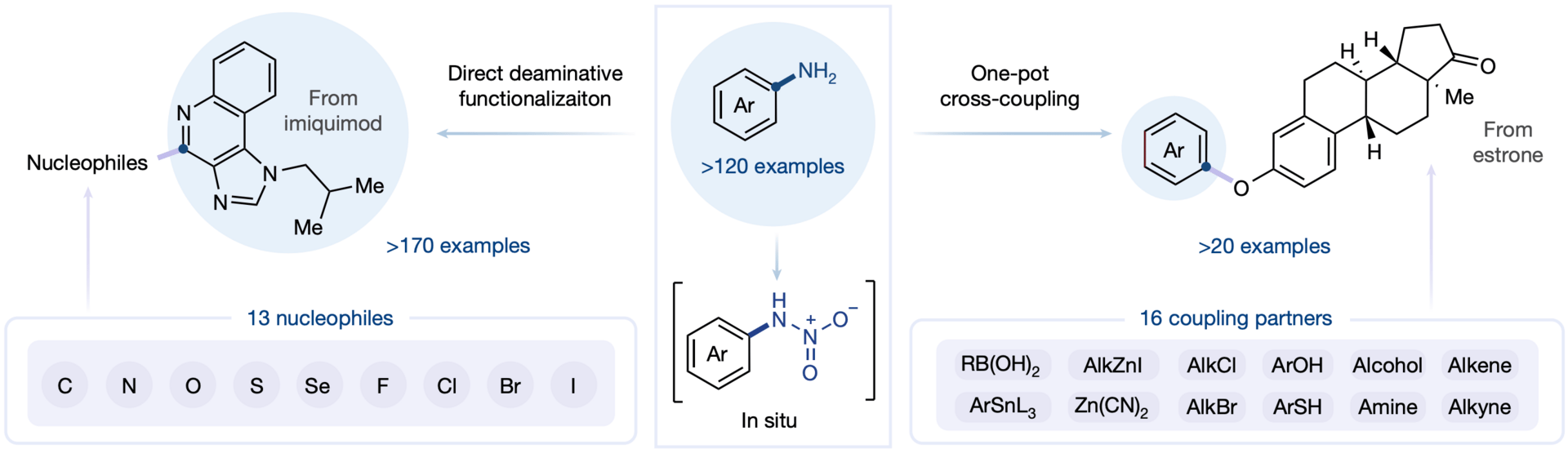
Amines are common in bioactive molecules, but traditional methods for converting aromatic amines often use explosive diazonium intermediates, posing safety risks. Here, the authors report a direct deaminative strategy through the formation of N-nitroamines, allowing the direct conversion of inert aromatic C−N bonds into an array of other functional groups, including C−X (C−Br, C−Cl, C−I, C−F, C−N, C−S, C−Se, C−O) and C−C bonds. This operationally simple, general protocol also establishes a unified strategy for one-pot deaminative cross-couplings by integrating deaminative functionalization with transition-metal-catalysed arylation.
#5
Deaminative Giese-type Reaction
P. Ma, Z. Cui & H. Lu*
Nat. Chem. 2025, 17, 1556–1564 (DOI: 10.1038/s41557-025-01888-8)

The authors present an approach to integrate nitrogen-atom deletion into the aza-Michael reaction, redirecting the classical pathway from C(sp3)–N bond formation to C(sp3)–C(sp3) bond construction. Leveraging commercially available O-diphenylphosphinylhydroxylamine (DPPH) as an efficient nitrogen-deletion reagent, this method enables a wide variety of primary aliphatic amines to serve as alkyl sources in couplings with structurally diverse electron-deficient olefins. This Giese-type reaction proceeds under mild conditions, achieves completion within 10 min and exhibits broad functional-group compatibility.
#6
One-Carbon Homologation of Alkenes
M. C. Grocott & M. J. Gaunt*
Nature 2025, 643, 130–138 (DOI: 10.1038/s41586-025-09159-9) 🔓

The authors report a catalytic one-carbon homologation process that is effective for many classes of alkene in simple and complex molecules. By leveraging the intrinsic reactivity of a new multifaceted allylsulfone reagent, a streamlined one-pot process, involving cross-metathesis and a fragmentation–retro-ene cascade, formally inserts a single methylene unit into the alkene chain. Among the applications of this process to several structurally and functionally complex molecules, this transformation was used to generate unexplored homologues of cyclosporine A. These homologues exhibit modulated pharmacological and biological properties and could provide promising leads as cyclophilin inhibitors, a target that has great potential in many disease areas.
#7
Deaminative Cross-Coupling of Amines by Boryl Radical β-Scission
Z. Zhang,† G. Lonardi,† T. Sephton,† Y. C. Guersoy, C. Stavagna, G. V. A. Lenardon, M. Bietti & D. Leonori*
Nature 2025, 647, 913–920 (DOI: 10.1038/s41586-025-09725-1) 🔓

The authors report a strategy that repositions native primary, secondary and tertiary amines as handles for cross-coupling. The platform relies on in situ activation through borane coordination and exploits a copper catalytic redox system that generates amine-ligated boryl radicals, which undergo β-scission across the C(sp3)–N bond to release alkyl radicals. These intermediates engage in copper-catalysed cross-couplings with a broad range of C-based, N-based, O-based and S-based nucleophiles. The method tolerates diverse amine classes, enables modular functionalization and supports late-stage diversification of complex drug scaffolds.
#8
Accelerating Medicinal Chemistry: A C(sp3)-Rich Fragment Toolbox for Redox-Neutral Cross-Coupling
J. Tsien,† Á. Péter,† X. Zeng, S. Wang, B. Jiang, M. A. Emmanuel, M. S. Oderinde, P. N. Bolduc, M. C. Nicastri, S. Dey, M. R. Collins, J. W. Lee, M. Bravo, P. F. Richardson, N. W. Sach, L. Bernier, M. D. Palkowitz, J. X. Qiao, Y. Kawamata & P. S. Baran*
Angew. Chem. Int. Ed. 2025, 64, e202517207 (DOI: 10.1002/anie.202517207)
Previously: ChemRxiv (DOI: 10.26434/chemrxiv-2025-2k4tc-v2) 🔓
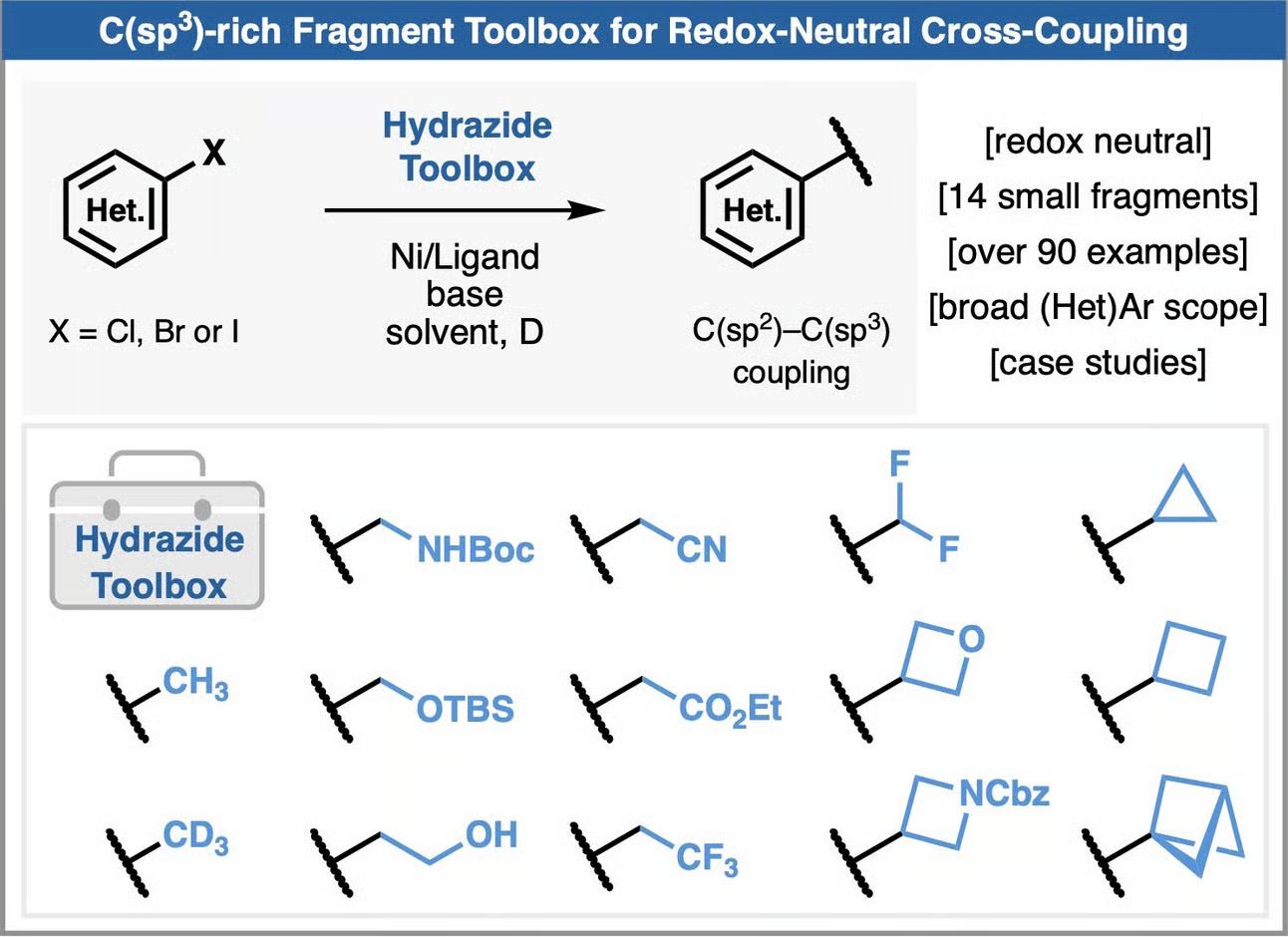
The authors introduce a toolbox of 15 sulfonyl hydrazide reagents to facilitate redox-neutral, nickel-catalyzed radical cross-coupling of 14 distinct small fragments onto (hetero)arenes under mild conditions. These crystalline, bench-stable reagents are straightforward to synthesize from accessible precursors and require no additional oxidants, reductants, or precious metals, offering a modular and operationally simple platform. Demonstrated across a diverse set of over 60 (hetero)aryl halides, the method exhibits exceptional substrate scope and functional group tolerance, accommodating complex, medicinally relevant scaffolds. Comparative studies with existing techniques underscore its advantages, including a 51% yield for trideuteromethylation of a MET kinase inhibitor precursor (vs. a precedented 14% via Kumada coupling) and a streamlined one-step cyclobutylation of an NLRP3 inhibitor intermediate at 41% yield (vs. a known <5% over a four-step sequence).
#9
Nitrate Reduction for Deaminative Suzuki–Miyaura Coupling of Anilines
C.-C. Li,† Á. Adorján,† M. Sofiadis, T. Schulte, J. Mateos, M. Rippegarten & T. Ritter*
Angew. Chem. Int. Ed. 2025, 64, e202504012 (DOI: 10.1002/anie.202504012) 🔓
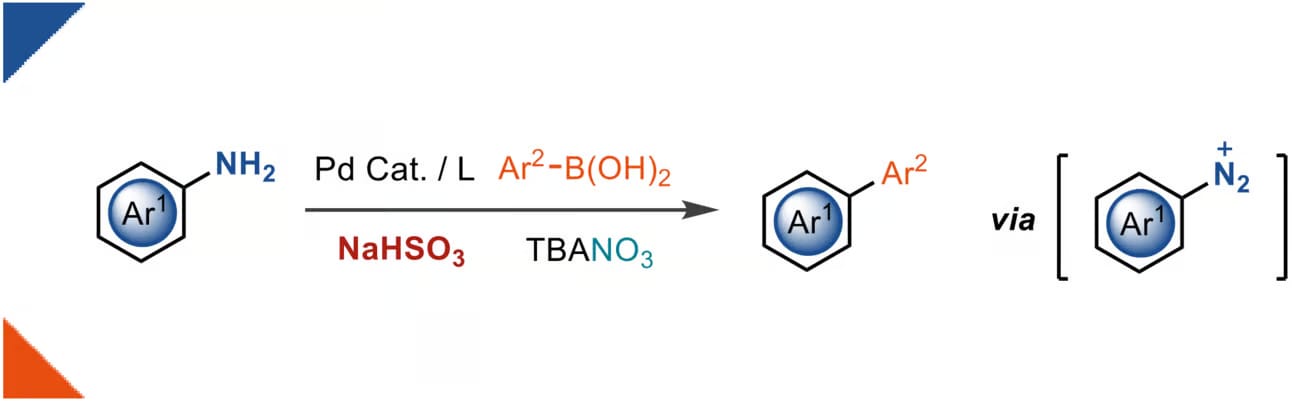
The authors present a deaminative Suzuki–Miyaura-type coupling of anilines with nitrate as a diazotization reagent, which integrates transition-metal catalysis with nitrate-based diazonium chemistry for the first time. The synergistic reduction of nitrate by bisulfite and boronic acids allows for both oxidative diazotization with low-valent transition metal redox transformations simultaneously. The reaction utilizes low-hazard, readily available starting materials and reagents. In comparison to previous diazonium-based Suzuki–Miyaura-couplings, the in situ oxidation of anilines by reduction of nitrate allows larger functional group tolerance.
#10
The Total Synthesis of (−)-Spiroaspertrione A: A Divinylcyclopropane Rearrangement Approach
W. Huang,† L. Pan,† H. Zhao, F. Schneider* & T. Gaich*
Science 2025, 390, 261–265 (DOI: 10.1126/science.adz7593)

The rise of multidrug-resistant pathogens poses a major threat to global health, with methicillin-resistant Staphylococcus aureus (MRSA) among the most challenging. One promising approach to overcoming resistance is using small molecules that resensitize MRSA to existing drugs. Here, the authors report the enantioselective total synthesis of one such promising candidate, (−)-spiroaspertrione A, a complex meroterpenoid of the andiconin family. The route features a stereoselective Diels-Alder cycloaddition, followed by a key divinylcyclopropane rearrangement forming the spirobicyclo[3.2.2]nonane core. Strategic late-stage functionalization of the compact cage architecture enabled access to the natural product and provided evidence for a plausible biosynthetic relationship with (−)-aspermerodione.

The Best Science Images of 2025
🔭 Best of 2025. Nature have just released their selection of the best science images of 2025, featuring “The Fall of Icarus”, an iconic image by astrophotographer Andrew McCarthy that captures skydiver Gabriel C. Brown as a perfect silhouette against the turbulent surface of the Sun. Dark sunspots and filaments, revealed through hydrogen-alpha imaging, form the dramatic backdrop. Months of meticulous planning, precise aircraft positioning, split-second jump timing, and a bespoke solar telescope were required to create the illusion, with multiple alignment attempts before McCarthy and Brown finally nailed the sequence.
NB: For those interested, the photograph’s title references the Greek myth of Daedalus and Icarus. Imprisoned on Crete by King Minos, Daedalus—the inventor who designed the Labyrinth to contain the Minotaur—fashioned wings of feathers and wax to escape with his son. Ignoring his father’s warnings to not fly too close to the sun or sea, Icarus soared too close to the Sun; the wax melted, and he fell into the sea.

Reply